
This step by step guide explains what naturally occurring radioactive materials in groundwater are, where they come from, how they move, how they are regulated, and practical steps small water utilities and well owners can take to manage risk. The focus is pragmatic: do the homework before you drill, design wells to avoid contamination where possible, use non-treatment approaches first, and choose treatment only after you understand the geology and water chemistry. The goal is to give you an actionable roadmap so you can make decisions that protect public health while minimizing long term capital and operating costs.
Step 1: Understand what NORM are and why they matter
Naturally occurring radioactive materials, commonly abbreviated as NORM, originate from the Earths crust. The most important geogenic radionuclides for groundwater are uranium, radium and radon. These elements are linked through radioactive decay: uranium is the long-lived parent that decays through a chain of daughter products, including radium and radon. Each compound has different physical and chemical behavior in the subsurface and different routes of human exposure.
Key reasons NORM matter for groundwater supplies:
- They are widely present in soils and rocks at varying concentrations. In many states they rank among the most common groundwater contaminants when surveys include both anthropogenic and natural sources.
- They pose health risks at elevated concentrations. The primary health concern for most NORM is an increased lifetime cancer risk from chronic exposure. Uranium also has kidney toxicity at higher concentrations.
- They are regulated, and compliance requires appropriate sampling, monitoring and sometimes treatment. Understanding the units and measurement techniques used for radioactivity is necessary to interpret results correctly.

What compounds behave differently and why it matters
- Uranium: usually dissolved in water and reported as micrograms per liter (ug/L). Uranium speciation and mobility depend strongly on redox and carbonate chemistry.
- Radium: typically a dissolved cation that can co-precipitate with carbonate minerals or exchange onto cation exchange sites.
- Radon: a gas produced in the decay chain. Radon partitions to air as water is used in homes, which creates an inhalation exposure route (for example during showering).

Step 2: Learn the regulatory framework and measurement units
Before assessing or treating a well, you need to know what regulatory thresholds apply and how they are measured.
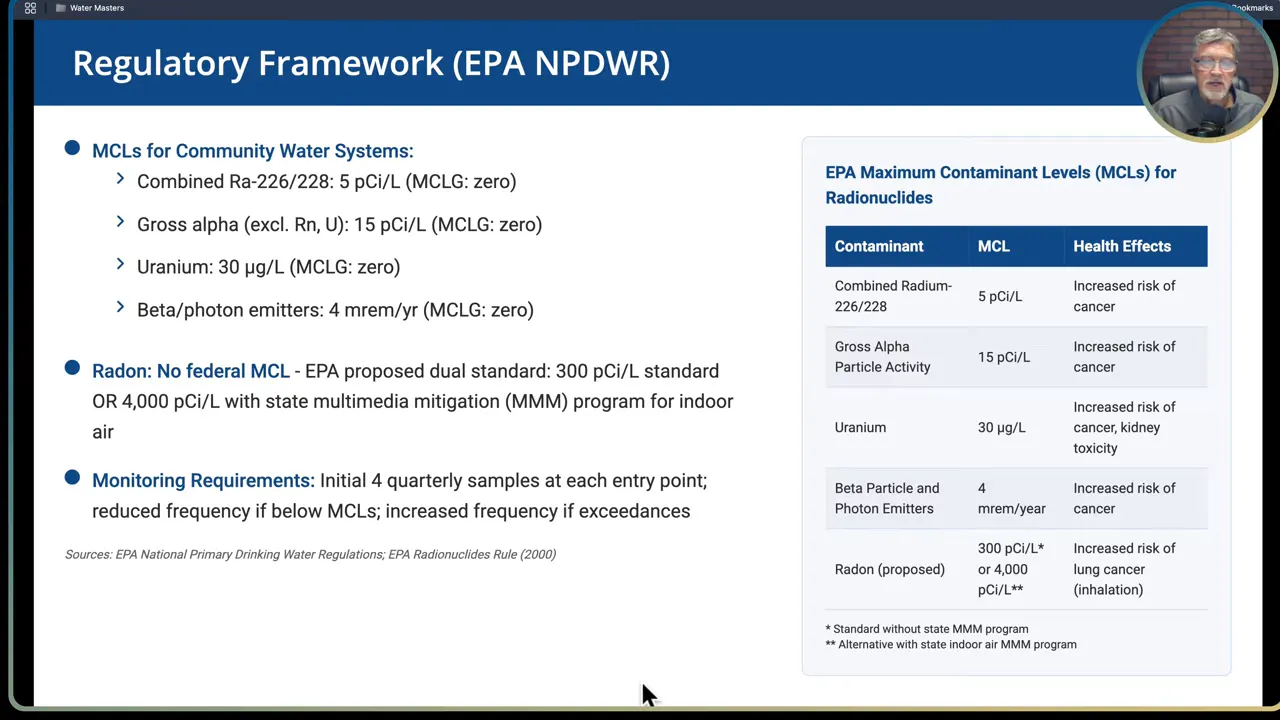
Common U.S. federal standards to be familiar with:
- Combined radium-226 and radium-228: Maximum Contaminant Level (MCL) = 5 picocuries per liter (pCi/L).
- Gross alpha particle activity (excluding radium and uranium): MCL = 15 pCi/L.
- Uranium: MCL = 30 micrograms per liter (ug/L).
- Beta and photon emitters: dose-based limit = 4 millirems per year.
Units and what they mean:
- Picocuries per liter (pCi/L) measure radioactive decay rate in a given volume and are commonly used for radium, radon and gross alpha measurements.
- Micrograms per liter (ug/L) measure mass concentration of dissolved uranium and are used where mass-based toxicology is important.
- Millirem per year (mrem/yr) express dose from beta and photon emitters. This is a radiation dose metric rather than concentration.
Sampling and monitoring rules (high level):
- Initial monitoring frequency for a new detection typically involves multiple quarterly samples at entry points to distribution systems. Frequency can be reduced when results fall below MCLs.
- Radon currently has no finalized federal MCL for water. The EPA proposed a dual approach (300 pCi/L or 4,000 pCi/L with a multimedia mitigation program) but that proposal was not finalized. For radon in water consider both drinking water ingestion and indoor air inhalation exposure pathways when deciding on mitigation.
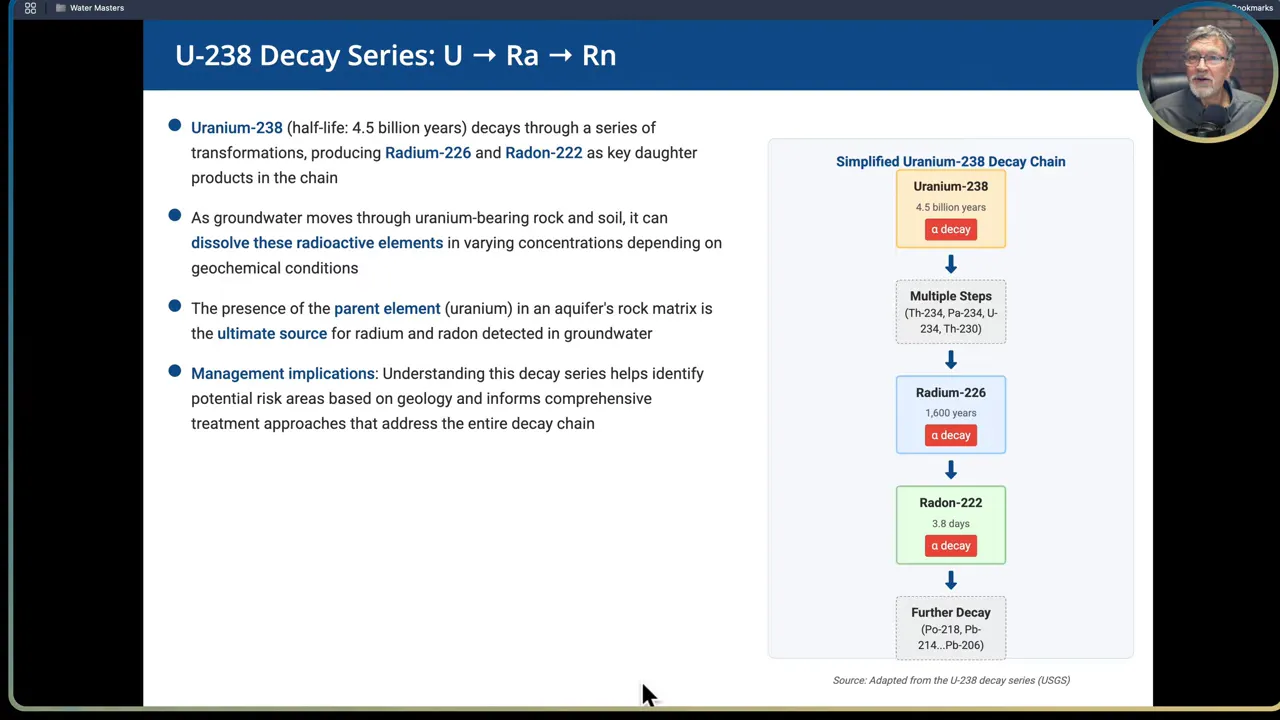
Step 3: Know the decay series and how parent-daughter relationships affect monitoring
Most NORM problems start with uranium-238 in rock. Uranium has an extremely long half-life of about 4.5 billion years, so it is essentially a stable background parent in geologic materials. As uranium decays it produces daughter radionuclides in a chain, notably radium-226 and radon-222 among others. Because these radionuclides are linked, detecting a daughter may indicate a parent source in the aquifer rock matrix.
Practical implications:
- Testing for a single radionuclide can miss related risks. If uranium is present in source rock you may also need to test for radium and radon as part of a full assessment.
- Radon is a gas and will behave differently from dissolved uranium or radium. If radon is present in groundwater, it can partition to indoor air when water is used in the home, creating inhalation exposure in addition to ingestion.
- Understand whether the measured radionuclide is a parent or daughter product. Management and treatment must take the decay chain into account to be effective.
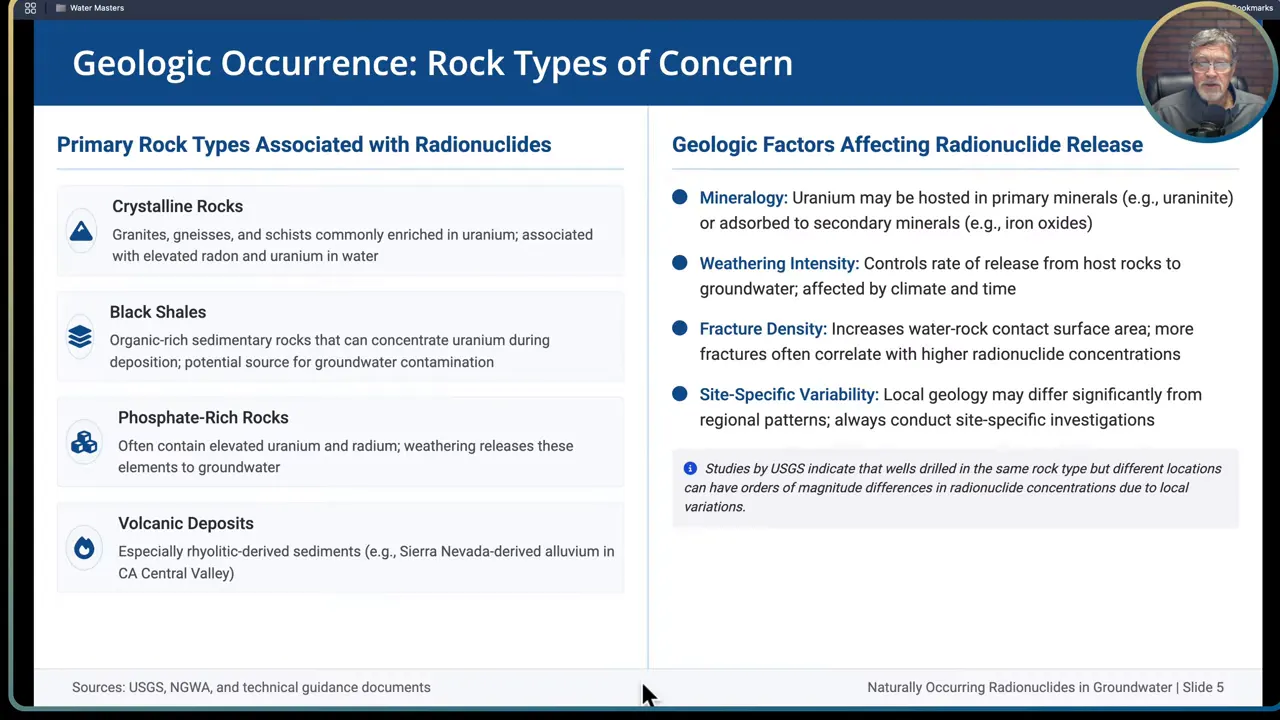
Step 4: Map geology and geographic hotspots before you drill
Geology largely sets the stage for NORM occurrence. Certain rock types and depositional environments are more likely to host elevated uranium and associated radionuclides.
Rock types and settings commonly associated with elevated NORM:
- Granites and metamorphic rocks: crystalline bedrock such as granites, gneiss and schists are often enriched in uranium and are associated with higher radon and uranium in groundwater. Some granites with pink feldspar show particularly high radon potential.
- Black shales and organic rich sediments: organic carbon binds uranium during deposition. Classic examples include regionally extensive black shales like the Chattanooga shale. Oil shales and organic-rich facies can concentrate uranium.
- Phosphate-rich rocks: phosphate deposits and phosphate-bearing strata frequently contain elevated uranium and radium.
- Volcaniclastic deposits and rhyolitic sediments: derived sediments from felsic volcanic sources can carry uranium into alluvium and valley fill.
Use available maps and datasets:
- EPA publishes radon potential maps by county which, while meant for indoor radon, correlate fairly well with geologic potential for groundwater radionuclides. These maps are a useful screening tool.
- USGS and state geological surveys often have data on local formations that concentrate uranium and shales with radioactivity. Review those sources during early planning.
Step 5: Do site specific testing because local variability is large
Even within a single rock type, radionuclide concentrations can vary by orders of magnitude over short distances. The only way to know what a particular well will produce is to do site specific investigation and testing.
Key actions to perform before completing a production well:
- Gather local well data and review prior testing results for nearby domestic wells, monitoring wells, or public supply wells.
- Examine geologic logs for mineral indicators such as uranium minerals or iron oxide zones that may point to a higher probability of radionuclides.
- Use radon and geological maps as a first pass to identify high risk areas where more detailed testing is needed.
When to tighten sampling and investigation
If the regional setting indicates elevated NORM potential or if known high uranium or radon exists nearby, plan a more conservative investigation. That means narrower vertical sampling intervals and more frequent analyses during drilling and testing. In practice the presenter recommends tighter sampling than a typical 50 foot screening interval when indicators point to high radionuclide variability.
Step 6: Understand the geochemistry that controls uranium mobility
Redox and carbonate chemistry are the dominant controls on uranium mobility in groundwater. Getting the geochemistry right is the single most important step to predicting where uranium will be dissolved and where it will be immobilized.
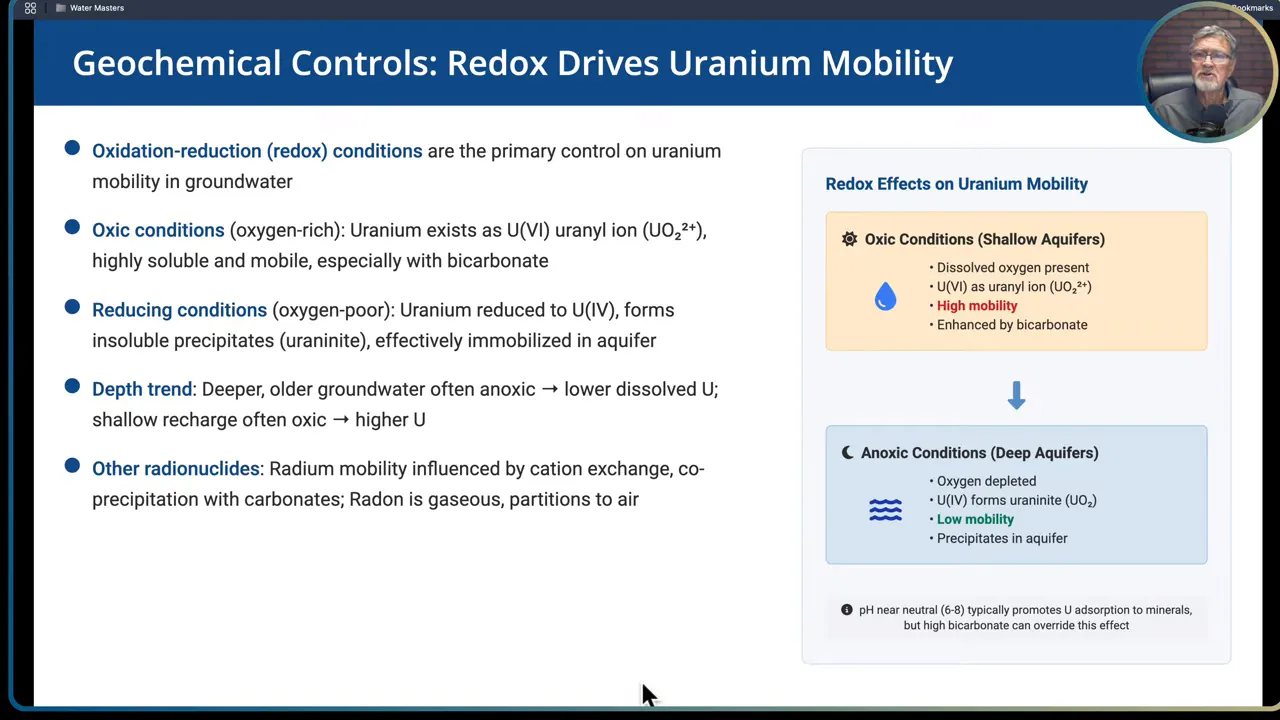
Redox controls
- Oxic conditions (oxygen rich): uranium exists mainly as hexavalent U(VI) in the form of the uranyl ion. U(VI) is highly soluble, especially when complexed by bicarbonate. Oxic shallow groundwaters are typically where dissolved uranium levels are highest.
- Reducing conditions (anoxic): uranium is reduced to U(IV), which precipitates as uraninite and other insoluble phases, effectively immobilizing uranium in the aquifer matrix.
Practical rule of thumb: shallow recharge water that is oxic and high in bicarbonate tends to carry dissolved uranium. Deeper, older, anoxic groundwater commonly contains lower dissolved uranium because U(IV) is immobile.
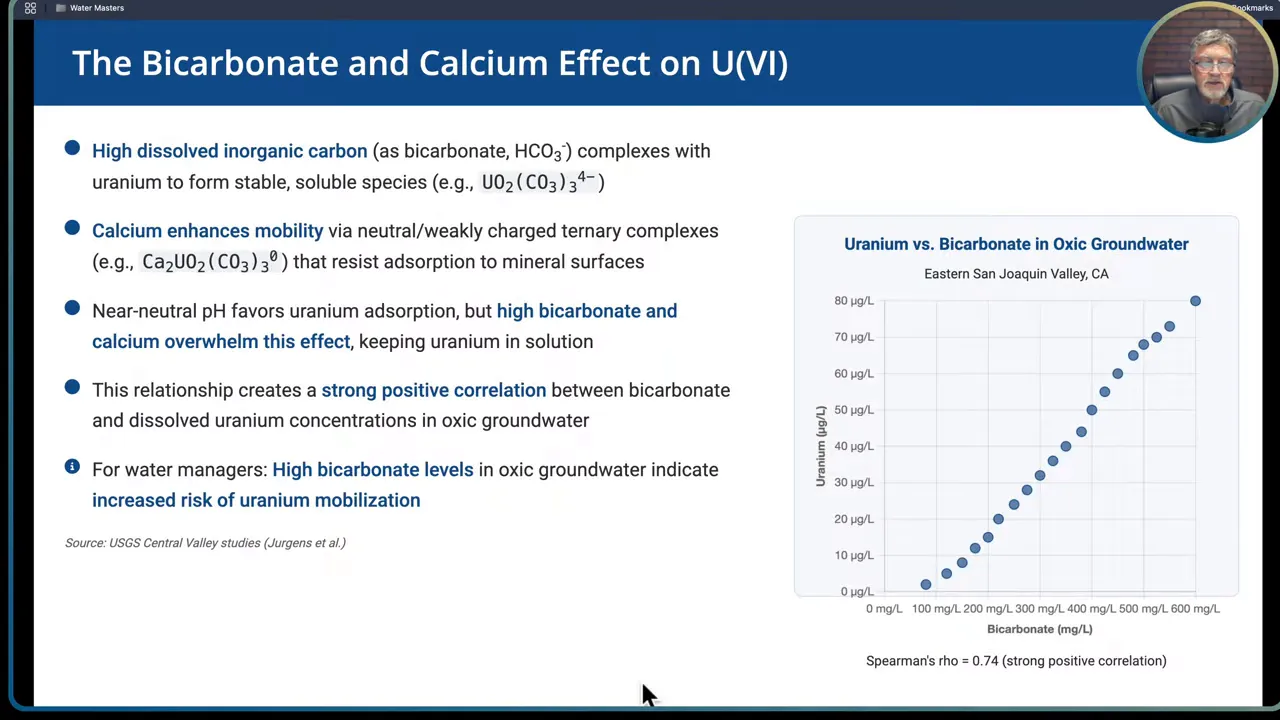
Carbonate and calcium effects
High dissolved inorganic carbon, primarily as bicarbonate HCO3-, forms stable soluble complexes with uranyl ions. Calcium can form ternary complexes with uranyl and carbonate that are neutral or weakly charged and resistant to adsorption on mineral surfaces. In short, bicarbonate and calcium can keep uranium in solution even at neutral pH.
Implication: a neutral pH (6 to 8) alone does not guarantee uranium will stick to solids. If bicarbonate and calcium are abundant in oxic water, uranium will remain mobile. Testing for background constituents like bicarbonate, calcium, dissolved oxygen, and ORP is essential to interpret uranium results and to select appropriate treatment.
Step 7: Recognize hydrogeologic and operational impacts
Pumping and land use can change flow patterns and water chemistry, altering the distribution of radionuclides over time. Two common operational drivers are intensive pumping and irrigation recharge.
- Intensive pumping: drawing high volumes from wells can create downward hydraulic gradients that pull shallow, oxic, high bicarbonate recharge water into deeper screened intervals. That can bring uranium down into public supply zones that were previously low in radionuclides.
- Irrigation recharge: irrigation return or recharge from agricultural fields commonly has elevated bicarbonate because of root respiration and microbial decay producing carbon dioxide which dissolves and increases bicarbonate. Over time this recharge can increase uranium mobilization in oxic settings.
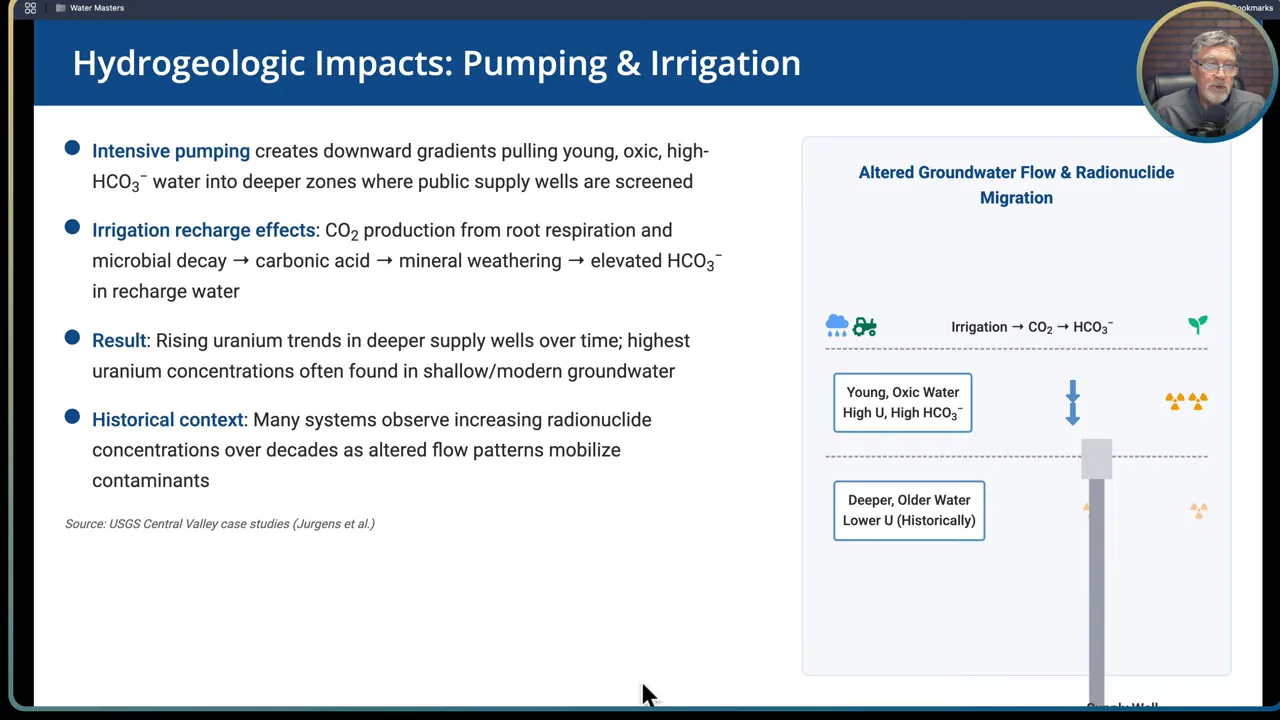
Result: many systems show rising uranium or radionuclide trends over decades as pumping and land use alter flow paths. That is why long term trend monitoring is critical; an aquifer that meets standards today can shift out of compliance as operations evolve.
Step 8: Prioritize well siting, vertical profiling, and completion design to avoid contamination
The most cost effective strategy is to avoid contaminant inflow into the well in the first place. Careful siting, test drilling, vertical profiling, and thoughtful completion dramatically reduce the need for expensive treatment later.

Pre-siting assessment
- Review local well logs and water quality data.
- Consult geologic and radon potential maps.
- Avoid locating wells down gradient from heavily irrigated areas if that pattern could bring high bicarbonate recharge into production zones.
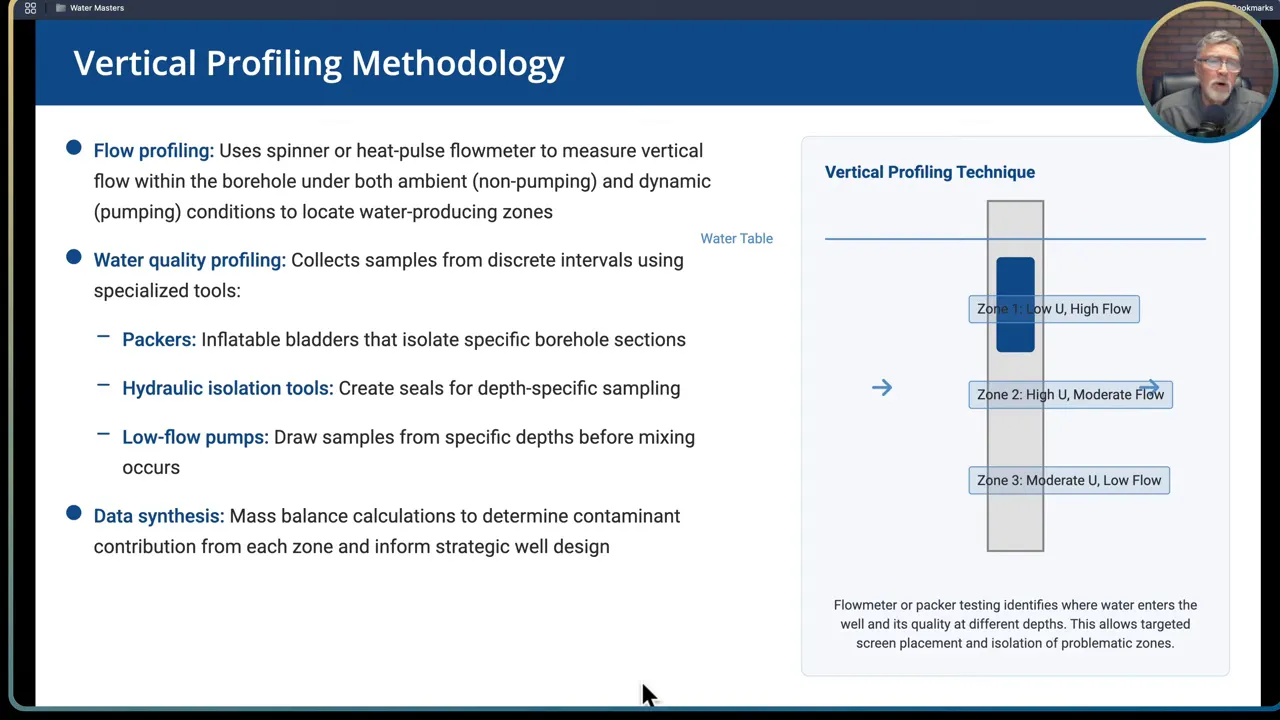
Test wells and vertical profiling
Vertical profiling is the workhorse for defensible well design. The steps are:
- Drill a test well or use a production-size test bore to access the targeted aquifers.
- Gather a detailed geologic log and note iron oxide zones, fractures, or mineralized layers that could host uranium.
- Perform flow profiling with a borehole flowmeter or other profiling tool to determine which depths contribute water and at what rates.
- Collect discrete water samples from the distinct contributing intervals for radionuclide and geochemical analysis.
- Run a mass balance to determine how blended flows from selected intervals will affect final concentrations and yield.
Mass balance and screen placement
Use the mass balance equation to decide which intervals to include in a final screened interval and which to exclude or seal off:
Cblend = (Q1*C1 + Q2*C2 + … + Qn*Cn) / (Q1 + Q2 + … + Qn)
Example from practice: Source A produces 5 ug/L uranium and Source B produces 45 ug/L uranium. If you blend 75 percent of source A with 25 percent of source B the blended concentration is
Cblend = 0.75*5 + 0.25*45 = 3.75 + 11.25 = 15 ug/L
The blended result of 15 ug/L is below the 30 ug/L uranium MCL and is therefore compliant. Blending is a legitimate, cost effective compliance strategy when sources and flows can be controlled and monitored.
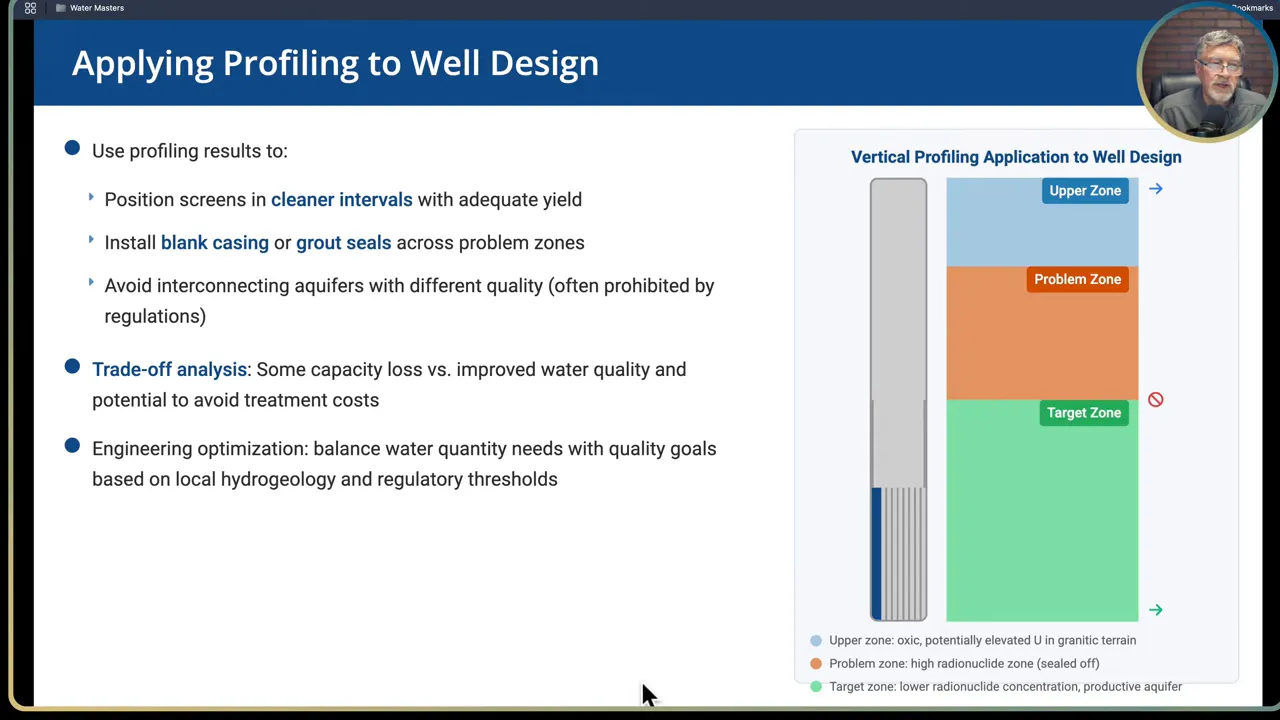
Well construction details to avoid cross contamination
- Install grout seals or blank casing across problem intervals to avoid downward or upward movement along the borehole. Grouting sections isolates contaminated intervals and prevents migration via the annulus or gravel pack.
- Avoid mixing aquifers of differing water quality in a single screened interval. Regulations often prohibit interconnection of separate aquifers due to contamination risks.
- Consider pump intake depth adjustments if small changes can bring the finished water into compliance without losing much capacity. Raising or lowering the pump is a low cost option compared with major construction.
Step 9: Modify existing wells and use non-treatment compliance options first
If an existing supply well shows elevated radionuclides, determine whether modifications can restore compliance before committing to treatment.

Options for modifying wells
- Vertical profiling and flow testing to identify offending zones.
- Pressure grouting specific intervals to isolate them from the screened interval.
- Installing a liner in older wells where grouting is impractical. Liners may be a useful retrofit in legacy wells but are not always the preferred long term solution.
- Reconfiguring pump intake elevation to draw more from cleaner zones when the problem is marginal and a small change can bring the well into compliance.
After modification, always perform pumping tests and water quality monitoring to verify success and communicate changes to the state primacy agency. If the well is corrected you may be able to return to normal monitoring frequencies rather than facing increased regulatory sampling.
Non-treatment compliance strategies
- Blending: Combine lower uranium sources with higher ones to achieve a blended concentration below the MCL. Blending requires reliable source flows and frequent sampling to ensure continued compliance.
- Source substitution: Drill new wells in cleaner parts of the aquifer and use those as primary sources while retiring or repurposing high radionuclide wells.
- Seasonal operations: If water quality varies predictably with season, operate affected sources only during lower risk periods and rely on cleaner sources at other times.

Step 10: Choose treatment when non-treatment options are infeasible
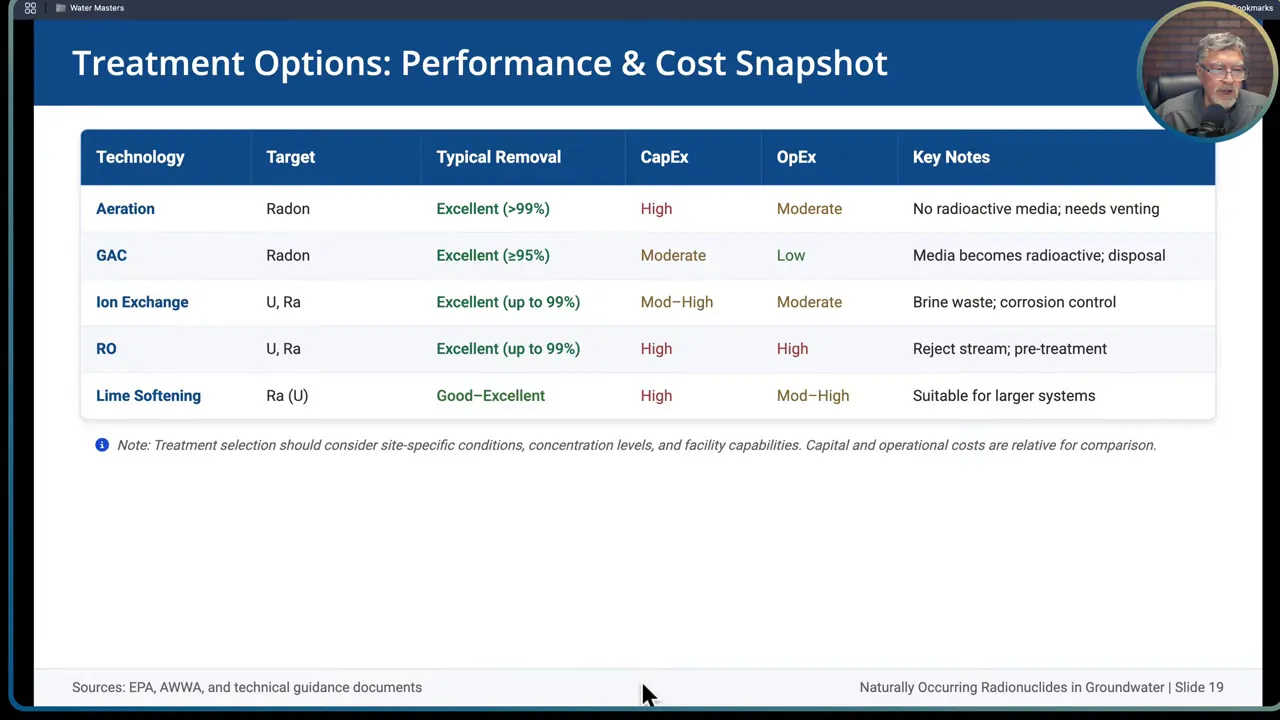
When source control, siting and blending cannot bring finished water below regulatory limits, treatment is necessary. Match the technology to the target radionuclide, water chemistry, waste handling capacity, operating budget and operator skill level.
Treatment options by radionuclide
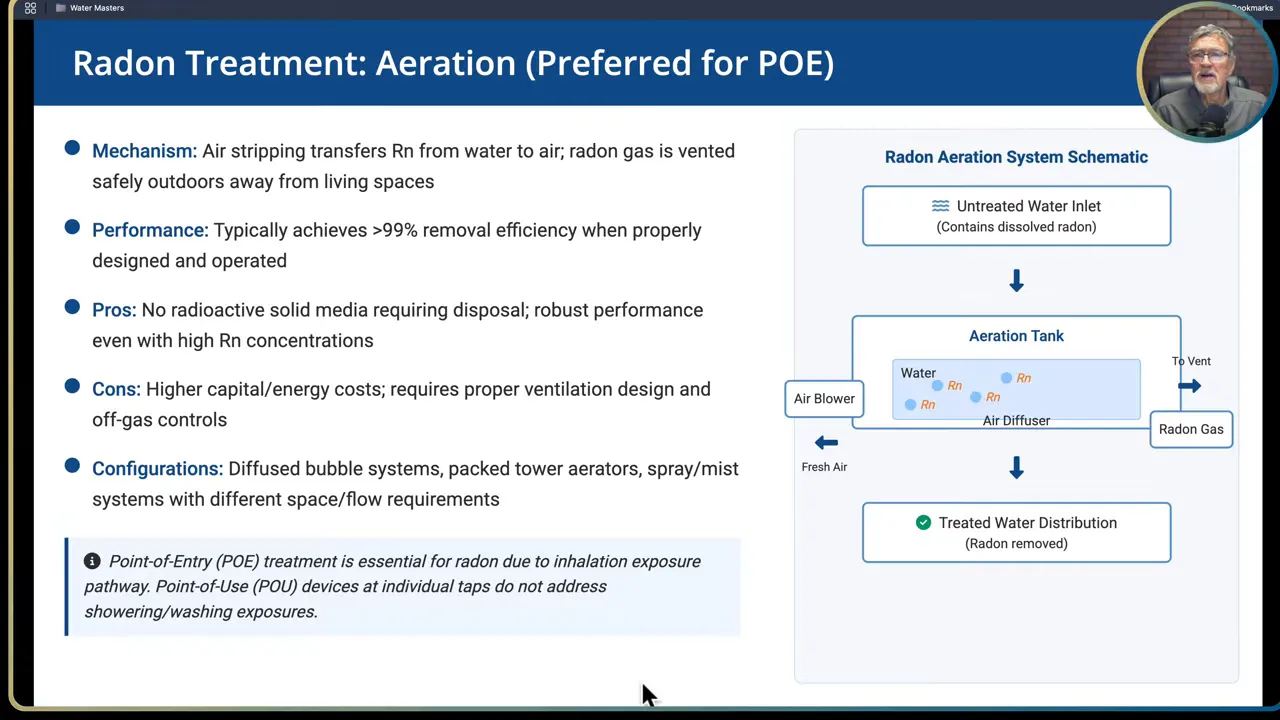
- Radon
- Aeration: preferred point of entry option. Transfers radon from water to air using packed towers, spray aerators or bubble aerators. Radon-laden air must be vented safely outdoors away from occupied spaces. Properly designed aeration can remove upwards of 99 percent of dissolved radon.
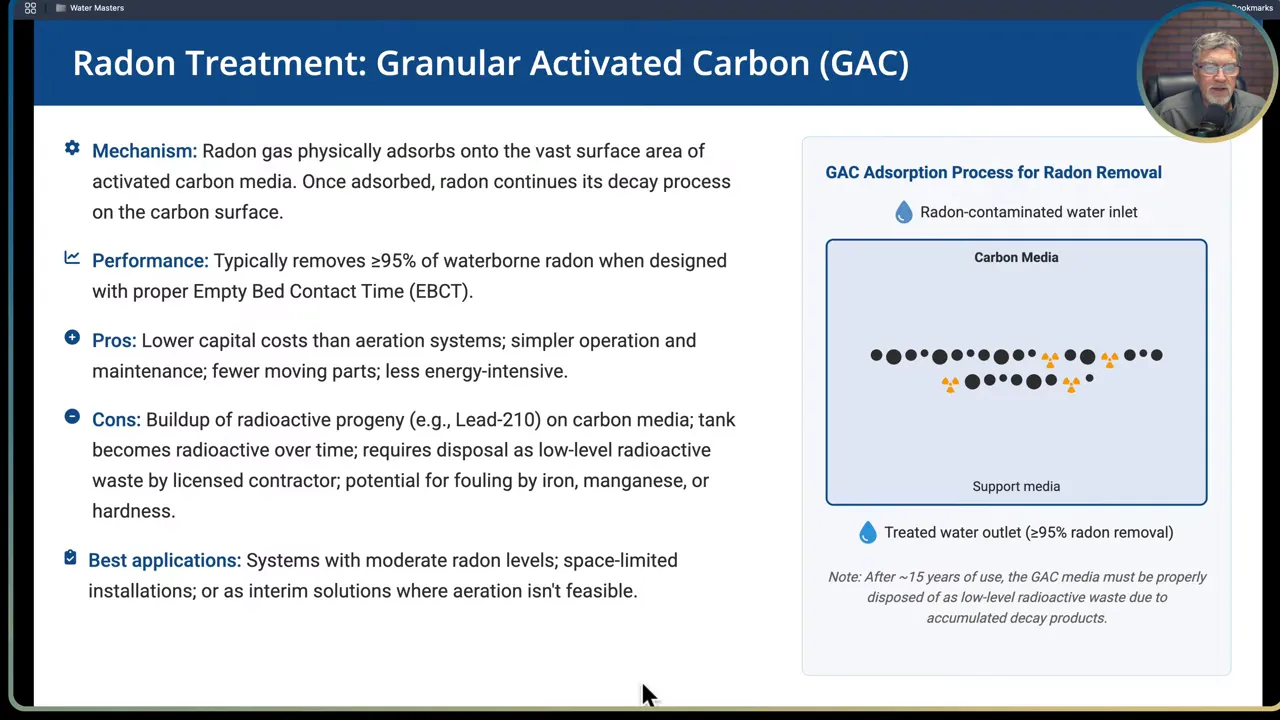
- Granular activated carbon (GAC): radon physically adsorbs to carbon. GAC can achieve around 90 to 95 percent removal when designed with adequate empty bed contact time. GAC has lower capital cost compared to aeration but produces radioactive solids that require licensed disposal when the media saturates with decay products.
- Uranium
- Anion exchange: very effective for U(VI) in oxic, high bicarbonate waters. Uses strong base anion resins to remove uranyl carbonate complexes. Removal efficiencies commonly 90 to 99 percent. Requires regeneration and handling of brine containing concentrated radionuclides, plus pretreatment for iron and manganese to avoid fouling.
- Reverse osmosis (RO) or membrane filtration: high rejection across micro to nano filtration membranes with removal up to 99 percent. RO produces a concentrate reject stream that is 10 to 70 percent of feed volume which requires disposal. Scaling control and pretreatment are necessary for high TDS or hardness waters.
- Lime softening: effective primarily for radium because radium co-precipitates with calcium carbonate. Under optimized conditions lime softening can achieve moderate uranium removal, but it is usually chosen when radium is the dominant target and for large systems with existing lime infrastructure.
- Iron or titanium oxide adsorbents: specialized media designed to bind uranium. These media provide good removal but generate solid radioactive wastes that require specialized disposal and typically cost more.
- Radium
- Cation exchange: radium is removed by cation exchange resins that replace sodium or potassium on the resin with radium. Typical removal efficiencies range from 85 to 95 percent. Cation exchange also co-removes calcium and magnesium, reducing hardness.
Practical considerations when selecting treatment
- Co-occurring constituents matter. Iron, manganese and hardness shorten resin and media life and increase maintenance frequency. Pretreatment is often required.
- Waste handling is a major driver. Regenerant brines from ion exchange, spent GAC, RO concentrates and sludge from lime softening contain concentrated radionuclides and are regulated. Plan disposal early and budget for it as an ongoing operating cost.
- Operator skill and O M: choices should match the operators capabilities. Some options require specialized knowledge to avoid breakthrough or scaling and to keep the system running safely.
- Life cycle costs: evaluate both upfront capital costs and long term operating expenses. RO and large aeration systems have high capex and opex compared with small GAC systems, but GAC media disposal and frequent replacement may still be costly depending on loading rates.

Examples of technology pros and cons
- Aeration: Pros are no radioactive solids to dispose of and robust performance. Cons are high capital and energy costs and the need for ventilation and air handling to avoid moving radon into living spaces.
- GAC for radon: Lower capital and simple operation. Cons include buildup of decay progeny (for example lead 210) that makes the media and vessel radioactive over time and requires licensed disposal. GAC is susceptible to fouling by iron, manganese or high hardness.
- Anion exchange for uranium: High efficiency for oxic, bicarbonate-rich waters. Requires regeneration and brine disposal. Pre-treatment for iron and manganese often required to maintain resin life.
- RO: Broad spectrum removal and very effective but creates concentrate waste and requires scaling control and energy. For small systems RO can be prohibitively expensive to operate.
Step 11: Operate, monitor, manage waste and maintain compliance
Treatment is not a set it and forget it activity. Radionuclide systems require careful operational monitoring, documented procedures, trained operators and a waste management plan. Establish Key Performance Indicators and a monitoring dashboard to detect trends early.

Recommended KPIs and routine monitoring
- Finished water radionuclide concentrations and percent removal.
- Process-specific metrics: pressure differentials, flow rates, EBCT for GAC, resin capacity and breakthrough indicators, membrane flux and recovery, and contact time in aerators.
- Resin regeneration frequency, brine volume and radionuclide loading of waste.
- Radiation surveys of vessels, spent media and areas handling waste to ensure worker and public safety.
- Post-treatment corrosion control: check pH, alkalinity and lead and copper to maintain compliance with the Lead and Copper Rule since some treatment steps can mobilize metals.
Waste management and disposal
Plan early for how to handle radioactive wastes generated by treatment processes. Typical options include:
- Concentration and evaporation of RO or anion exchange brine to produce a smaller mass of solid that can be disposed of in licensed facilities.
- Removal and licensed disposal of spent GAC, resins or adsorptive media. Spent media often qualifies as low level radioactive waste requiring specialized transport and licensed facilities.
- Solidification and stabilization of concentrates prior to disposal when required by state regulations.
Check state and federal regulations early. Your state primacy agency or a licensed low level radioactive waste contractor can advise on approved disposal pathways and permitting needs. Factor permit lead times and recurring disposal costs into your financial planning.

Personnel, training and documentation
- Develop Standard Operating Procedures for operation, monitoring and maintenance.
- Ensure operators receive training specific to radionuclide treatment and radiation safety.
- Consider maintenance service contracts for proprietary or complex equipment to reduce risk of misoperation and to ensure timely maintenance.
- Keep detailed records for compliance audits and to support continual improvement of the system.
Step 12: Review a real world example and the core takeaways
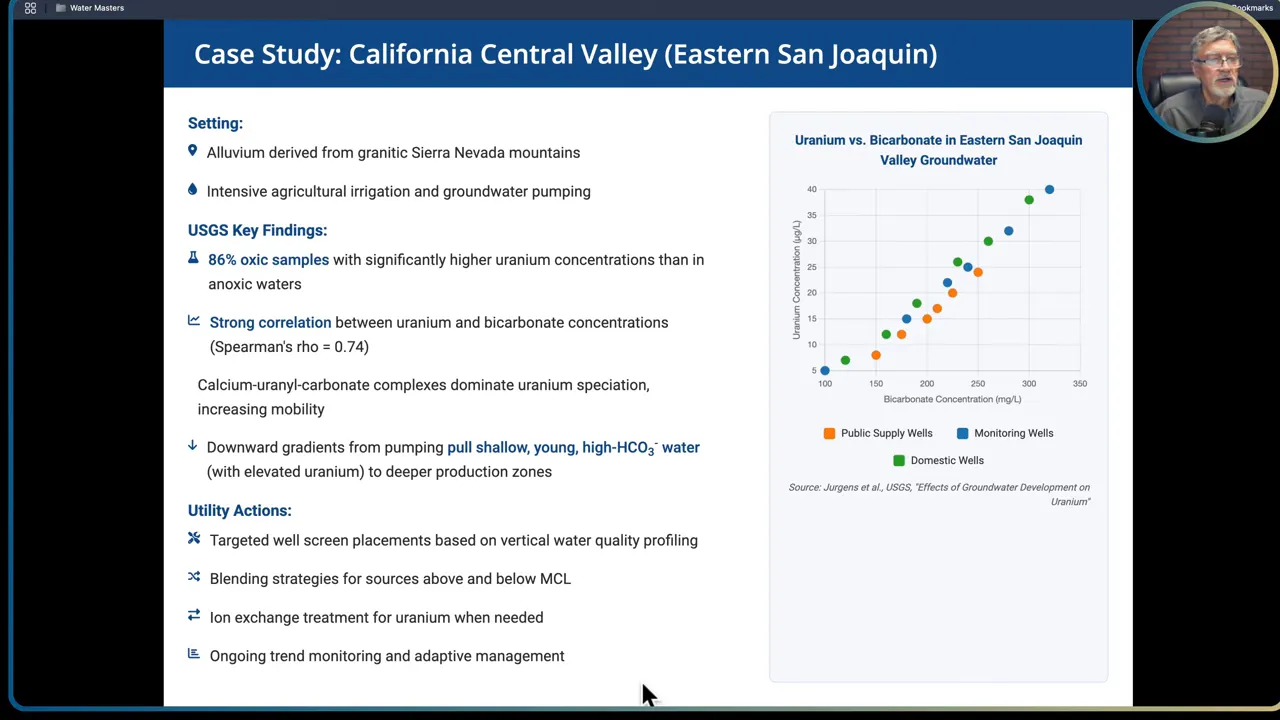
Case study summary: Eastern San Joaquin Valley, California. Source sediments derived from granitic Sierra Nevada rocks in a region with intensive agricultural irrigation and groundwater pumping. USGS study findings included:
- Oxic samples had significantly higher radium concentrations than anoxic samples. This matches the broader principle that oxygen rich, bicarbonate-laden shallow water is more likely to carry uranium and radium.
- Strong correlation between bicarbonate and uranium in oxic groundwater. In oxic settings bicarbonate concentrations can be nearly predictive of uranium concentration.
- Pumping created downward gradients that drew shallow, high bicarbonate water into deeper production zones, increasing radionuclide concentrations at supply wells over time.
- Utilities addressed the issue by targeting well screen placement with vertical profiling, applying blending strategies where possible, and using ion exchange for wells where non-treatment controls were insufficient. They used ongoing trend monitoring and adaptive management to maintain compliance.
Key takeaways
- Geology sets the stage: Understand rock types and formations before you drill. Granites, black shales, phosphate rocks and certain volcanic-derived sediments are higher risk.
- Geochemistry and operations control mobility: Redox state, bicarbonate, calcium and pumping practices determine whether uranium stays bound in rock or dissolves into groundwater.
- Do your homework ahead of time: Map, sample and profile before you commit to a production well. A well sited and completed correctly can save decades of treatment expense.
- Design wells to avoid high uranium and radium zones: Use vertical profiling and mass balance calculations to place screens in cleaner intervals. Grout out problem zones and avoid mixing aquifers.
- Start with blending and source management: Non-treatment options often cost much less over the life of the system than treatment options and should be prioritized if feasible.
- Select treatment aligned with waste and O M capacity: If treatment is required choose technologies that match your operators skills, waste handling capability and budget. Remember that waste disposal is a recurring cost.
- Monitor and adapt: Establish KPIs, track trends, and use predictive maintenance to avoid surprises. Long term operations and waste handling must be planned from day one.
Quick checklist for a small water utility starting a NORM assessment
- Gather local geology, radon maps and prior well data.
- Plan a test well with full geologic logging.
- Conduct vertical flow profiling and depth-discrete water sampling for radionuclides and geochemical parameters (dissolved oxygen, bicarbonate, calcium, pH, ORP, iron, manganese).
- Run mass balance blending calculations and evaluate screen placement options.
- If modification is chosen, design grout seals or liners and plan post-modification verification pumping tests.
- If treatment is required, select technology based on contaminant species, co-contaminants and waste management capacity, and secure necessary permits.
- Establish KPIs and a monitoring program for finished water and process performance. Train staff and document procedures.
- Implement waste handling and disposal procedures with licensed contractors if necessary.
Managing naturally occurring radioactive materials in groundwater is a multidisciplinary task that blends geology, hydrogeology, geochemistry, engineering and operations. For small utilities the emphasis should be on prevention, careful design, and cost effective solutions that minimize lifetime operating burdens. The more time you spend investigating and designing wells upfront, the fewer surprises and lower the lifetime cost of compliance.
If you are planning a well program or facing an existing radionuclide exceedance, follow the step by step process in this guide: assess geology, profile vertically, apply non-treatment options when possible, and then select treatment only when needed with a clear waste management plan in place. That approach will protect public health while keeping long term costs under control.


