
Arsenic in groundwater is one of those problems that quietly affects communities around the world. It is common, often naturally occurring, and it can be hard to predict without the right testing and planning. If you manage a small water system, run a domestic well, or are responsible for planning new source development, this step by step guide walks through what you need to know: how arsenic is released, where it is likely to occur, how to reduce it at the source, and what treatment and residuals management options work best in practice.
Step 1: Understand the health risk and regulatory limits
Arsenic is a toxic carcinogenic trace element and a metalloid. It can cause skin lesions and multiple cancers including lung, bladder, and kidney. The EPA MCL is 10 micrograms per liter and the public health goal is zero.
Start here: arsenic is not just another inorganic parameter to check off your sampling sheet. It is toxic at low levels and a long-term drinking water concern. The U.S. Environmental Protection Agency enforces a maximum contaminant level (MCL) of 10 micrograms per liter or 10 parts per billion. That was lowered from the prior federal standard of 50 micrograms per liter in 2006. The public health goal—an unenforceable ideal—is zero.
Key statistics to keep in mind:
- National detection: Detections greater than 1 microgram per liter are reported in more than 50 percent of wells sampled across the U.S.
- National occurrence of exceedances: About 7 percent of wells in principal aquifers exceed the MCL of 10 micrograms per liter.
- Higher-risk regions: In the western U.S. roughly 16 percent of wells exceed the MCL, especially in basin-fill aquifers and closed basins.
- People affected: Estimates place millions of people relying on groundwater impacted by arsenic at various levels; planning and treatment decisions should assume arsenic could be present unless testing shows otherwise.
Because of health risk and regulatory attention, small systems must treat or manage arsenic reliably. Monitoring and frequent sampling will often be required if you are near the MCL. The regulatory community expects certified lab analysis, proper methods, and sampling at the entry point to the distribution system.
Step 2: Know the geologic sources and where arsenic commonly occurs
Arsenic is a geologic element. It occurs in over 200 minerals and is commonly associated with sulfide ores. A key mineral to remember is arsenopyrite and arsenic-bearing pyrite, which often coexist with iron minerals in many rock types.
Common geologic settings where arsenic is released to groundwater include:
- Volcanic and granitic rocks, where arsenic-rich sulfides are part of the rock matrix.
- Meta-sedimentary rocks such as black shales and slates with fine-grained organics and sulfide minerals.
- Basin-fill and alluvial deposits, especially closed basins and dry lake beds where evaporation concentrates solutes.
- Deltaic and alluvial plains such as large river valleys where young sediments contain arsenic.
- Glacial and fluvial deposits, where buried organic material and reducing horizons can generate conditions that mobilize arsenic.
Examples matter. In the western United States, basin-fill aquifers like those in the Central Valley of California and Mojave-region basins commonly show elevated arsenic. In parts of India and Bangladesh, young alluvial sediments and groundwater with long residence times produce some of the world’s most severe arsenic problems. Even within a single valley, arsenic can be variable — a ridge or fault can separate low and high arsenic zones; on one side of a valley you may get compliant water and on the other side water that must be treated or blended.
Practical takeaway: map and research local geology before you locate a well. Regional groundwater quality datasets, geologic maps, and prior well data are essential early inputs to a responsible drilling program.
Step 3: Learn the mobilization mechanisms — why arsenic becomes waterborne
Understanding arsenic in groundwater is really a chemistry and process problem. The element is often present in the solid phase but can be released to water when geochemical conditions change. Key mobilization mechanisms include:
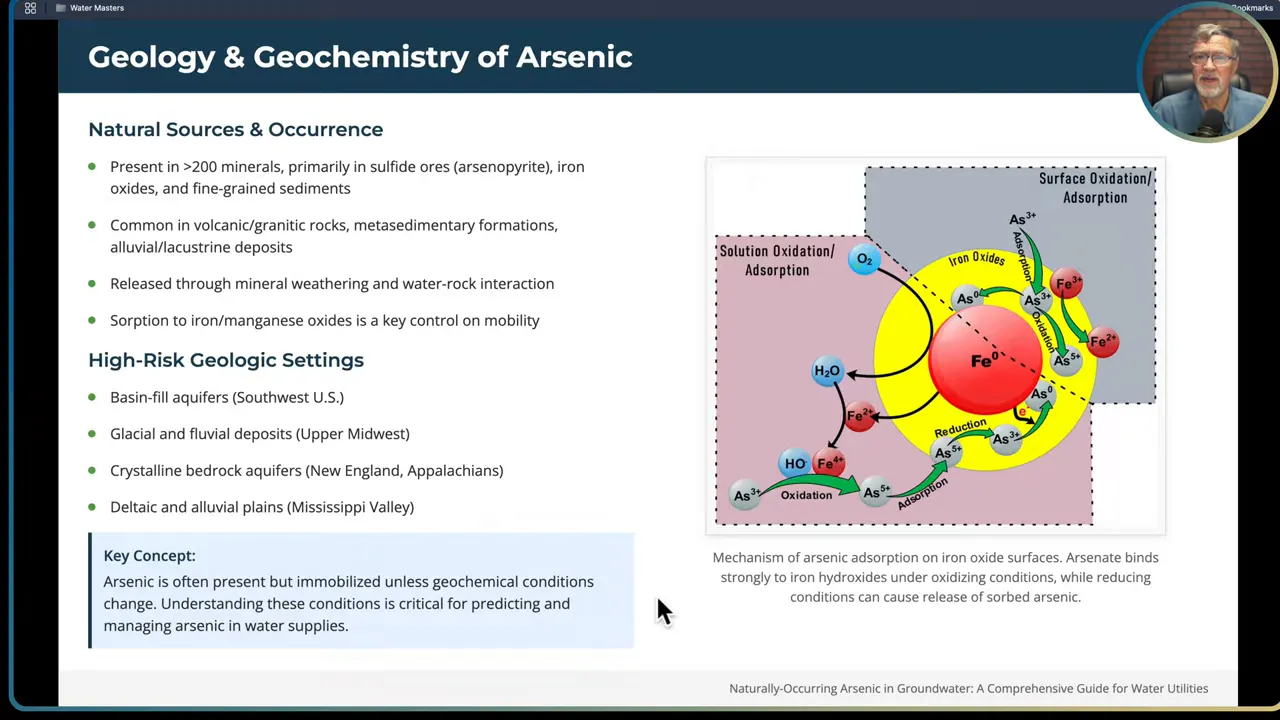
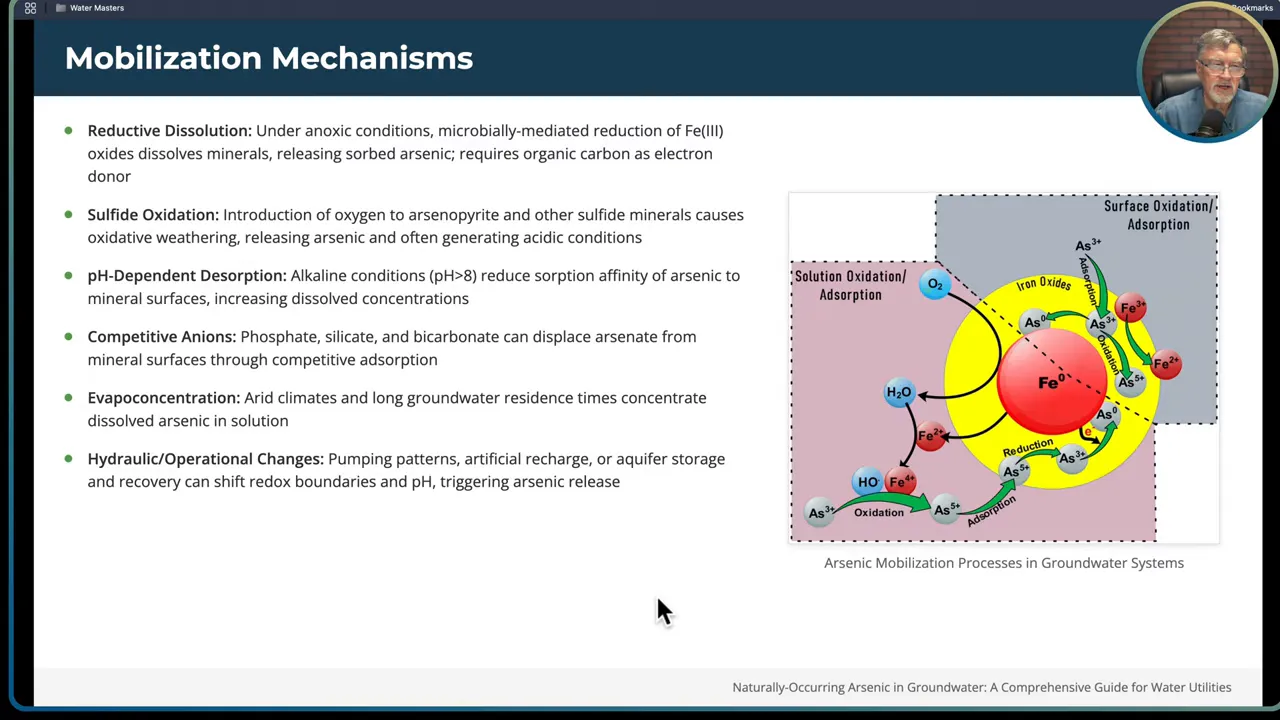
- Reductive dissolution. Under low oxygen conditions, microbially mediated reduction of iron oxyhydroxides releases iron and any arsenic sorbed to them. Organic carbon fuels the microbes and acts as an electron donor. In many aquifers, iron-reducing bacteria are the unseen drivers that dissolve iron oxides and release arsenic previously locked onto those surfaces.
- Sulfide oxidation. When oxygen or other oxidants reach arsenic-bearing sulfide minerals such as arsenopyrite, oxidative weathering liberates arsenic and often generates acidic conditions. This can happen when shallower oxygenated water mixes with deeper reduced groundwater or when pumping patterns alter flow paths.
- pH-dependent desorption. Alkaline conditions (pH greater than about 8) reduce arsenic affinity for mineral surfaces. High pH can therefore drive arsenic from solid phase into solution.
- Competitive anions. Phosphate, silica, and bicarbonate compete with arsenate for sorption sites on mineral surfaces. In waters with elevated phosphate or silica, these anions can displace arsenate and increase dissolved arsenic concentrations.
- Evapoconcentration and long residence time. In arid closed basins, evaporation concentrates dissolved arsenic. Long groundwater residence times also allow gradual release and accumulation of arsenic in the dissolved phase.
- Hydraulic and operational changes. Pumping pattern changes, recharge operations, or artificial storage and recovery can shift redox boundaries or pH, leading to sudden mobilization of arsenic.
These mechanisms mean arsenic occurrence is dynamic — it is controlled by the redox environment, pH, and competing chemistry. A well that has produced low arsenic for decades can start showing higher levels if pumping increases, recharge changes, or seasonal chemistry shifts. This is why monitoring and periodic reassessment are not optional.
Step 4: Identify arsenic species and why speciation matters for treatment
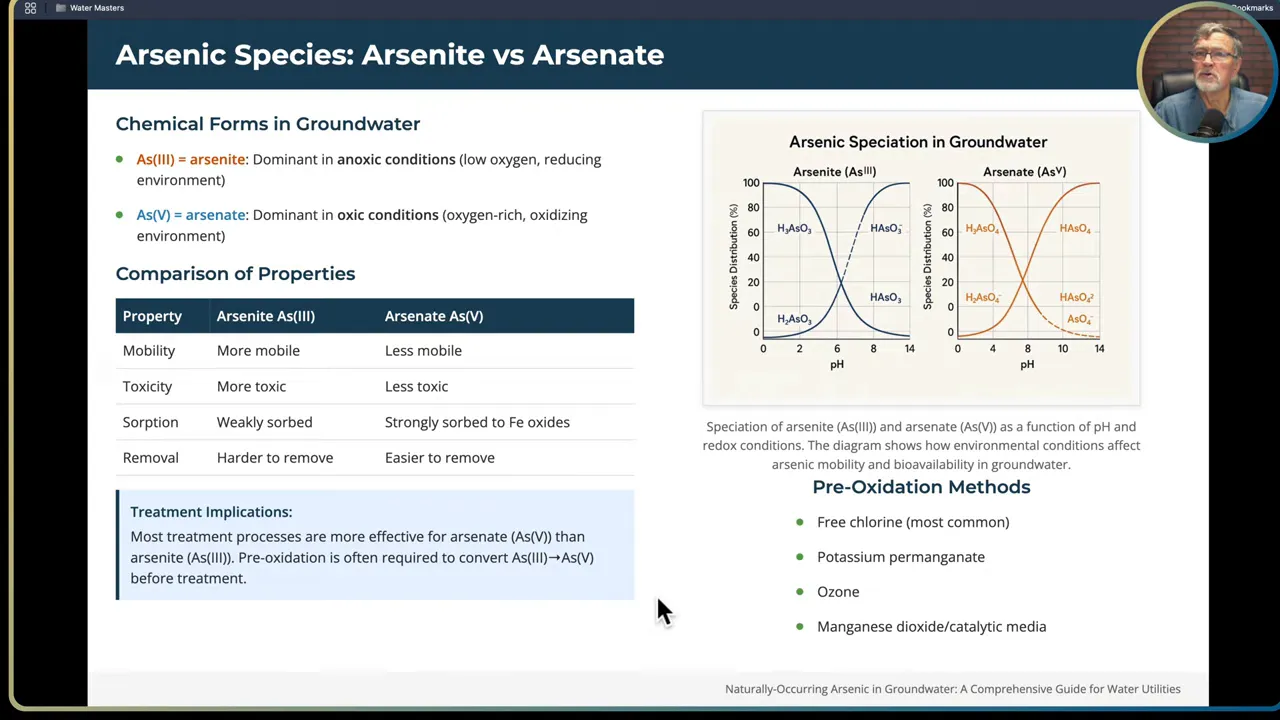
Arsenic in water exists mainly in two oxidation states: arsenite As(III) and arsenate As(V).
- Arsenite As(III) is the reduced form. It dominates under anoxic, reducing conditions. It is more mobile, less strongly sorbed to iron oxides, and more toxic than As(V). It is harder to remove using adsorption or coagulation.
- Arsenate As(V) is the oxidized form. It dominates under oxidizing conditions. It is less mobile, strongly sorbed to iron oxides, and easier to remove with common water treatment methods.
Why this matters for operations: most removal processes, including coagulation with iron salts and adsorption on iron-based granular media or activated alumina, remove As(V) more effectively than As(III). Therefore preoxidation is often required to convert As(III) to As(V) before the main removal step. The most common preoxidants in water systems are free chlorine, potassium permanganate, ozone, or catalytic media such as manganese dioxide.
Always get speciation data when you do baseline testing. Total arsenic is important, but knowing the ratio of As(III) to As(V) informs whether you need oxidation in the treatment train and what residuals handling will look like. Many labs offer speciation testing; budget for it early in your assessment.
Step 5: Conduct a baseline assessment and build an initial monitoring plan
Before you design a treatment system or finalize a source selection, do a proper baseline assessment. This is not optional if you intend to comply with regulations and keep your community safe.
Baseline assessment checklist:
- Compile historical data: existing well logs, prior arsenic results, neighboring well chemistry, and regional water quality datasets.
- Collect new samples: measure total arsenic and speciated arsenic (As(III)/As(V)). Send samples to a certified lab using EPA-approved methods.
- Screen for interferences: measure iron, manganese, silica, phosphate, bicarbonate, alkalinity, pH, dissolved oxygen, and total organic carbon. These interact with arsenic removal processes.
- Decide sampling frequency: initial monitoring is typically quarterly or monthly depending on results; if you are near the MCL you will likely be required to sample more frequently and meet regulatory monitoring schedules at entry points to the distribution system.
- Track documentation: consumer confidence reports and public notices must include arsenic results and any MCL exceedances. Keep detailed records of laboratories, methods, and chain of custody.

Small systems may qualify for variances or exemptions if full centralized treatment is economically infeasible, but expect that regulatory teams will require a timeline and credible plan for returning to compliance. Variances are not automatic and are often difficult to obtain.
Step 6: Vertical profiling and source control — minimize the problem at the well
Whenever possible, manage arsenic at the source. Vertical profiling is the most cost-effective opportunity to reduce arsenic inflow to a treatment system or to avoid treatment altogether.
Vertical profiling concept:
- Sample the well across discrete intervals while drilling or from an existing well using packers, low-flow sampling, or hydraulic isolation tools.
- Measure both static (ambient) and dynamic (pumping) conditions. Some zones flow only under pumping and contribute disproportionately to production and arsenic load.
- Use flow measurement tools: spinner flow meters, heat-pulse flow meters, or tracer tests to quantify contributions by interval.
- Run a mass balance to determine which intervals supply the bulk of flow and arsenic load. This informs where to place screens, seals, and where you might seal off high-arsenic zones.
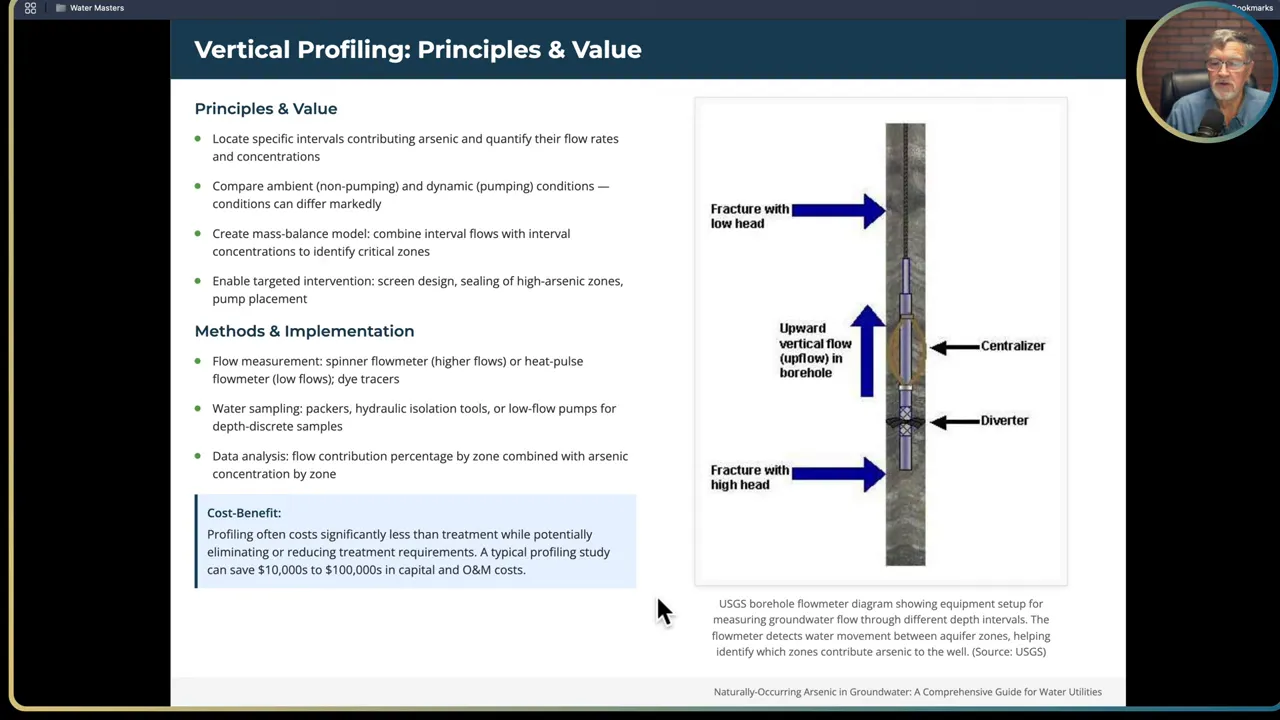
Practical profiling tips:
- Sample at a minimum every 50 feet for a general profile; in known arsenic districts use tighter intervals for higher-resolution data.
- Prefer modern downhole tools over old “zone testing” methods. Contemporary equipment can deliver high-definition profiles that tie chemical data to lithology and flow contribution.
- Drill a pilot well in new projects. A pilot well is a modest investment compared to a full production well plus a treatment plant that you may not need if you can avoid arsenic by better well placement and design.
When vertical profiling is done well, a properly designed screened interval and packer/seal layout often reduce arsenic significantly. Shorter screened intervals placed in low-arsenic horizons are usually the best long-term solution.
Step 7: Site selection, well design, and how to modify existing wells
Well construction is where design meets practice. Small differences in casing depth, annular seal placement, gravel pack extent, and screen length determine whether you have to manage arsenic chemically or can control it physically.
Design and construction recommendations:
- Site selection: use geologic and regional groundwater quality data to avoid known high-arsenic formations when feasible.
- Screen strategy: prefer multiple short discrete screens placed in low-arsenic zones identified by vertical profiling. Avoid long continuous screens that straddle high- and low-arsenic intervals.
- Casing and annular seals: install deeper steel casing and competent annular seals to isolate shallow reducing zones. Proper grouting materials and techniques are essential to prevent crossflow between strata.
- Gravel pack: be careful where the gravel pack extends. A gravel pack extending to the surface can provide a flow path outside the casing and bypass seals, allowing contaminated shallow water to travel into deeper screened zones.
- Pump placement: set the pump intake in a low-arsenic interval if practical. Pump placement affects how the well draws from different zones while producing.
- Pilot testing: drill test wells and run a pilot vertical profile to confirm where the best low-arsenic production zones are before committing to full production well construction.

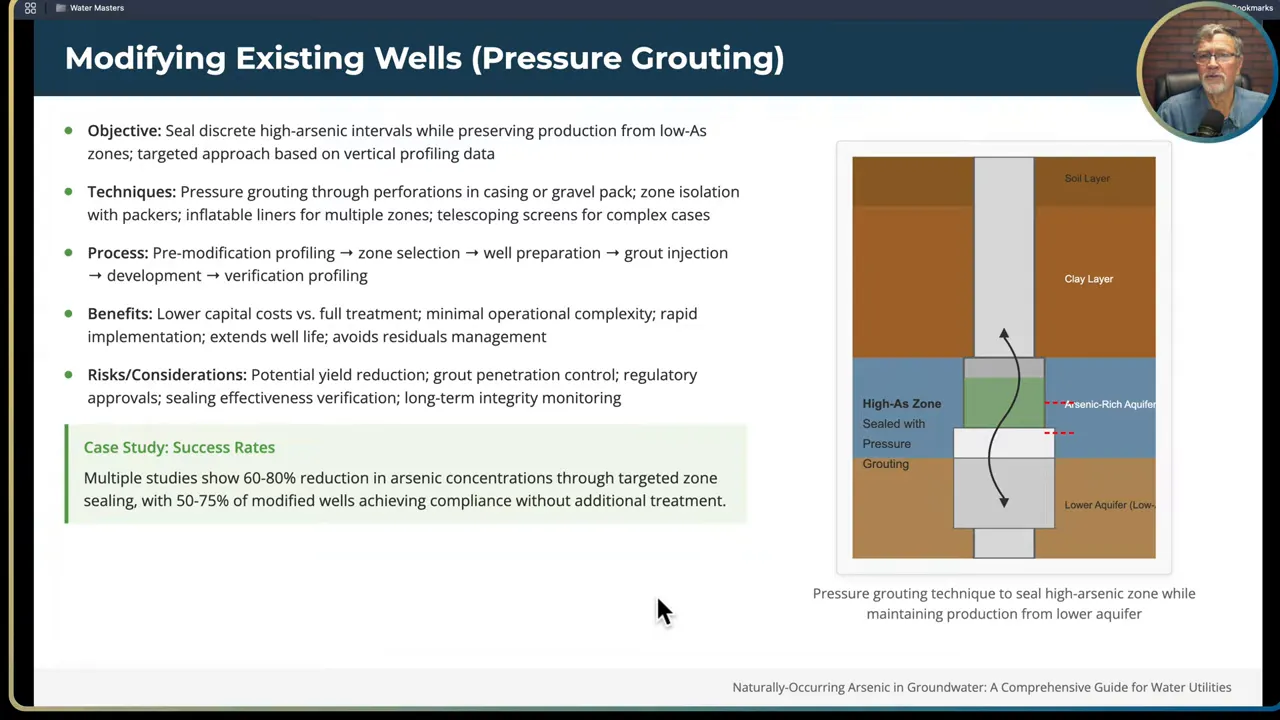
Modifying existing wells:
- If an existing well becomes contaminated by arsenic due to changing aquifer conditions, you can sometimes isolate the problem by sealing off specific screen intervals. This is usually done with packers or by selective grouting.
- Isolation grout is installed above and below the target zone, generally into an intervening clay or low-permeability layer. Squeeze cementing techniques can seal problematic screens.
- Be careful: sealing off a zone reduces production. Always balance desired water quantity versus arsenic reduction using a mass balance and production needs analysis.
Step 8: Blending strategies — a non-treatment approach when you have mixed sources
Blending can be a cost-effective and rapid route to compliance when you control multiple sources with varying arsenic concentrations. The strategy averages arsenic concentrations from wells so the delivered water is below the MCL.
How blending works:
- Identify low-arsenic and high-arsenic sources in your system and their sustained yields.
- Calculate flows and arsenic mass to produce a blended concentration at or below your target (commonly less than the MCL with a safety margin).
- Implement operational controls (valves, well scheduling, flow meters) to manage fractional contributions from each source.
- Monitor frequently — regulators often require robust monitoring where blending is used to meet compliance.
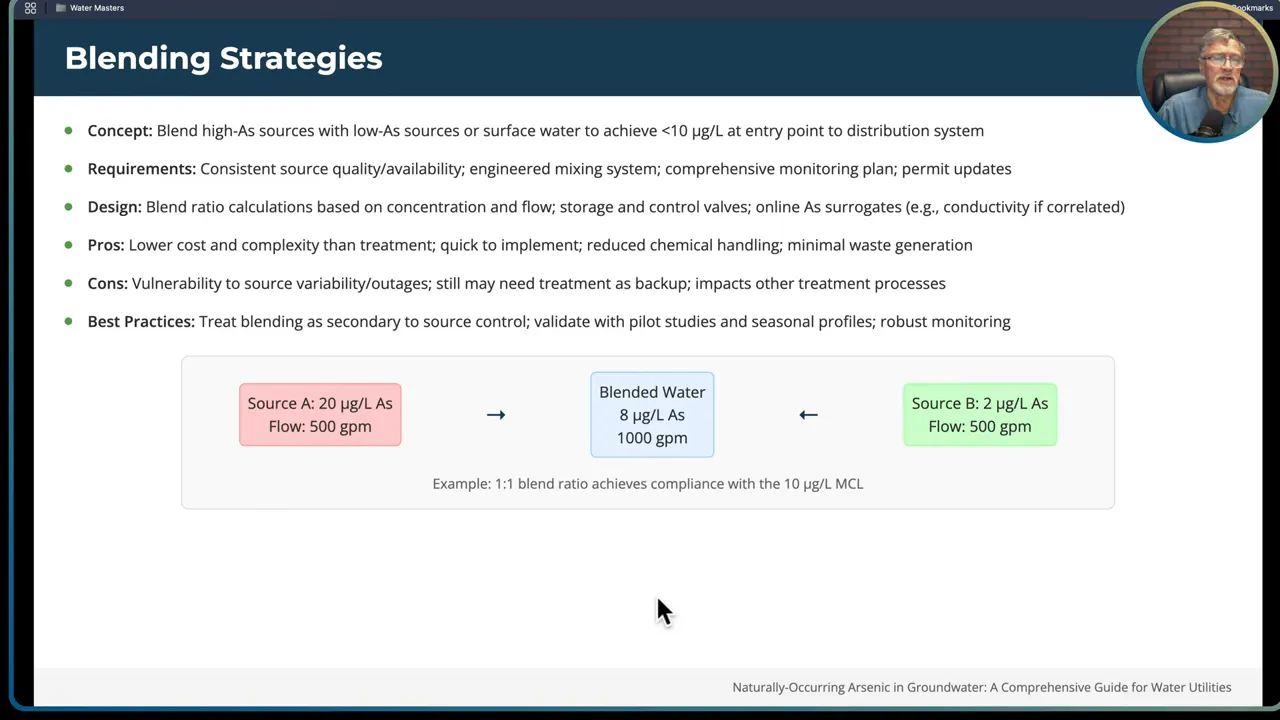
Blending example with simple math:
Suppose:
- Source A: 20 µg/L arsenic, capacity 500 gallons per minute (gpm)
- Source B: 2 µg/L arsenic, capacity 500 gpm
Combined flow = 500 + 500 = 1000 gpm
Mass-weighted concentration = (20 µg/L * 500 gpm + 2 µg/L * 500 gpm) / 1000 gpm
= (10,000 + 1,000) / 1000 = 11 µg/L
This blend yields 11 µg/L — still just above the MCL. To reach below 10 µg/L you would either need a larger contribution from the low-arsenic source or reduce the high-arsenic source contribution. For example, with Source B at 1000 gpm and Source A at 500 gpm, the blend becomes:
Mass-weighted concentration = (20*500 + 2*1000) / 1500 = (10,000 + 2,000) / 1500 = 8 µg/L
That meets the MCL with a reasonable buffer.
Advantages and limitations of blending:
- Pros: lower capital and operational costs compared to full treatment, quick implementation, less chemical handling, minimal residuals.
- Cons: vulnerable to variability in source quality and outages, may require more frequent sampling, and can complicate downstream treatment (if present) due to changing chemistry.
Always validate blending with pilot testing, seasonal sampling (to capture variability), and conservative operational rules to prevent excursions.
Step 9: Treatment technologies — which to choose and how they work
If source control and blending cannot reliably keep arsenic below the MCL, you must pick a treatment path. Below are the primary technologies used by small systems, with strengths, limitations, and operational considerations.
Coagulation and filtration (iron-based)
How it works: Preoxidation converts As(III) to As(V). Iron salts such as ferric chloride are dosed to form iron hydroxide floc. As(V) co-precipitates or adsorbs to iron hydroxide and is removed through solids separation such as rapid sand filtration, multimedia filtration, or clarifier/filtration sequences.
Key operating points:
- Optimal pH for maximum arsenate removal is typically 6.5 to 7.5.
- Iron-to-arsenic mass ratios often fall between 20:1 and 40:1; jar testing helps determine the correct dose for your water.
- Contact time and proper mixing are needed to allow adsorption/co-precipitation before filtration.
- Backwash solids handling and sludge disposal are critical downstream considerations.

Advantages and challenges:
- Advantages: co-removes iron and manganese sometimes present with arsenic, robust for variable water quality, established technology for small to medium systems.
- Challenges: chemical handling, sludge generation requiring proper disposal, and residuals may be hazardous depending on TCLP results.
Adsorptive media (granular ferric hydroxide, hybrid media, activated alumina)
How it works: packed beds of media adsorb arsenate from water as it passes through. Media can be granular ferric hydroxide (GFO), iron-impregnated resins, or activated alumina.
Operational points:
- Arsenic must be in the As(V) form for effective adsorption; preoxidation may be required.
- Empty bed contact times typically range from 3 to 6 minutes for effective removal.
- Lead/lag vessel arrangements provide breakthrough protection and predictable service life monitoring.
- Competing anions such as phosphate, silicate, and sulfate reduce media capacity by 30 to 50 percent; pre-treatment may be necessary.
- When media is exhausted it must be disposed of or regenerated. Regeneration with caustic is possible for some media but many spent media end up as waste that must be tested under the TCLP procedure for hazardous classification.

Pros and cons:
- Pros: simple operation, modular skids suitable for small systems, limited footprint.
- Cons: media cost, disposal complexity if hazardous, performance sensitive to competing ions and pH.
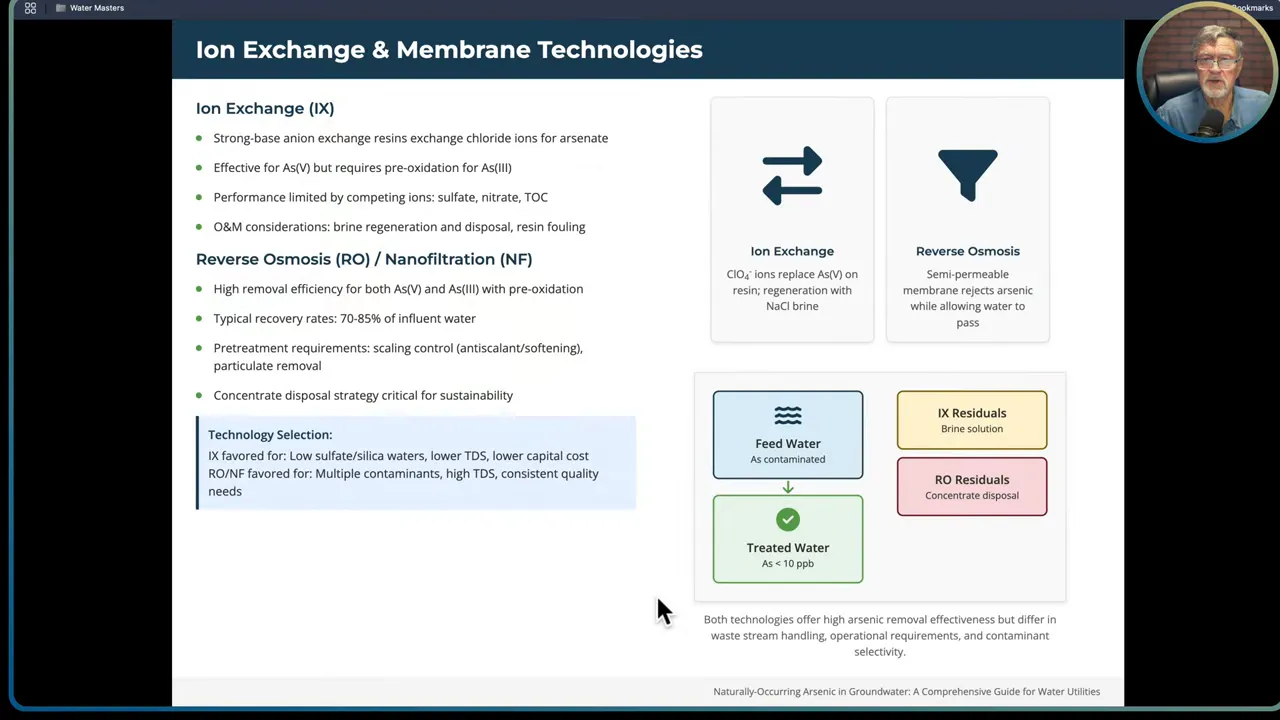
Ion exchange and membrane technologies (RO/NF)
Ion exchange resins can remove arsenate effectively under favorable water chemistries. Resin exchanges anions such as chloride for arsenate. Reverse osmosis (RO) and nanofiltration (NF) offer high removal efficiency for both As(III) and As(V) when combined with preoxidation, with RO giving the highest removal.
Key points:
- Ion exchange works best in waters with low sulfate and silica and lower total dissolved solids. Resin exhaustion occurs based on competing ions and resin capacity, and spent regenerant brine becomes a disposal issue.
- RO and NF require pre-treatment to control scaling and protect membranes. Typical recoveries are 70 to 85 percent; concentrate disposal is a long-term cost and sustainability challenge.
- Capital and operational costs for RO are typically higher than adsorption media but RO is favored when multiple contaminants need to be removed or when very consistent high-quality effluent is required.
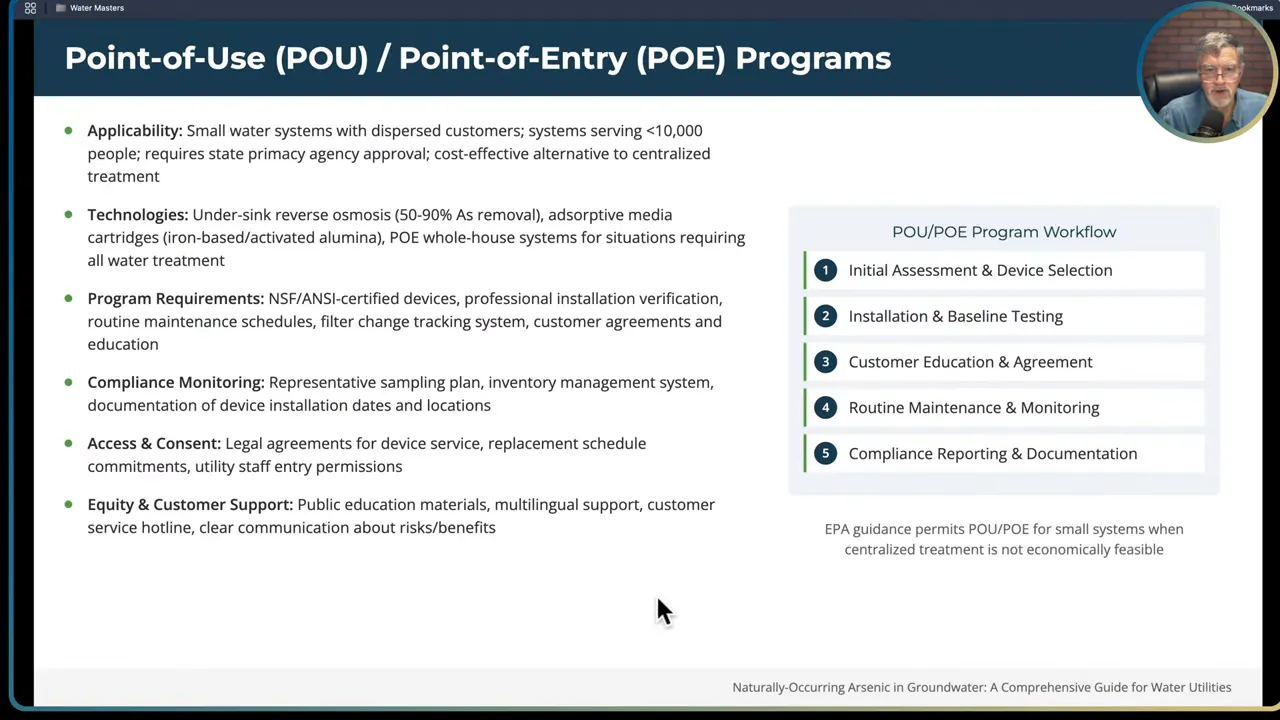
Point of Use and Point of Entry systems
Under exceptional circumstances small systems pursue point-of-use (POU) or point-of-entry (POE) devices installed at customer taps. These systems are usually cartridge adsorptive filters, small RO units, or ion exchange devices.
Considerations and cautions:
- POU/POE can be effective for individual households and very small systems, but they introduce management burdens: installation, ongoing monitoring, filter change-outs, and verification sampling.
- If the water system provides and maintains POU units for customers, that arrangement can lead to legal and liability issues if maintenance lapses and users are exposed to arsenic. Clear agreements and documented responsibilities are essential.
- The EPA allows POU/POE for small systems where centralized treatment is not economically feasible, but the system must document feasibility and implement monitoring and maintenance protocols.
Step 10: Residuals management — plan for the waste stream before you start treatment
Removing arsenic from water concentrates it somewhere else. Whether it is filter backwash sludge, spent media, ion-exchange resins, or RO concentrate, plan for residuals from day one. Residuals management is a recurring operational cost and may define your treatment choice as much as capital cost.
Common residual types:
- Coagulation-flocculation sludge that contains iron, arsenic, and other contaminants.
- Spent adsorptive media and ion exchange resins.
- RO/NF concentrate brine.
- Backwash wastewater containing suspended solids and adsorbed arsenic.
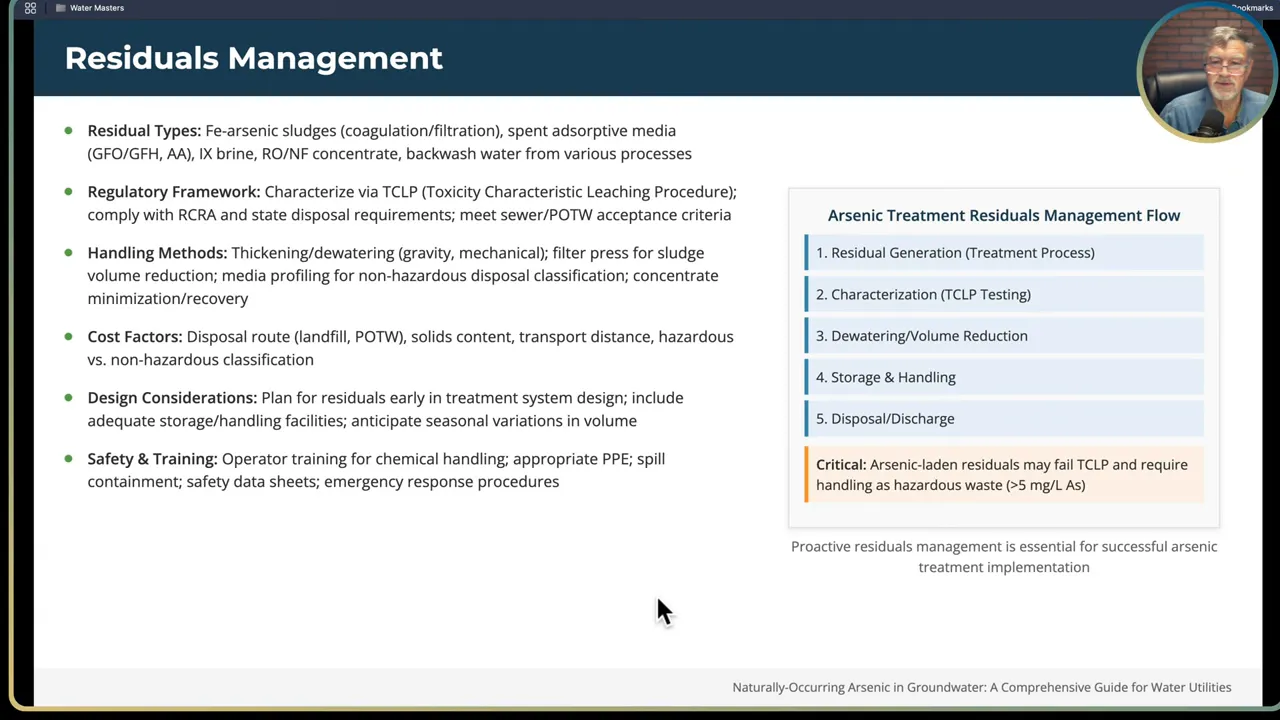
Regulatory disposal requirements:
- Perform a TCLP test (Toxicity Characteristic Leaching Procedure) on representative residual samples. TCLP determines whether the waste leaches hazardous concentrations under prescribed test conditions.
- If residuals fail TCLP, they must be managed and disposed of as hazardous waste following manifest and permitted disposal facility requirements. That substantially increases cost and logistics.
- If residuals pass TCLP, they may be disposed of under nonhazardous rules, but proper documentation and accepted facilities are still required.
Minimizing residuals volume:
- Dewatering, drying, or evaporation can reduce volumes and produce a drier waste form easier to transport and dispose of. Dry solids are often less expensive to dispose of than liquid brines.
- Consider thickening and volume reduction equipment in your plant layout and lifecycle cost model.
Cost planning: residual transport distance, facility acceptance fees, and hazardous waste management significantly affect life cycle cost. Factor these into your funding application and operational budget.
Step 11: Implementation roadmap — pilot test, fund, train, and operate
Treating arsenic is not a one-off construction project. It is a staged program requiring testing, pilot demonstration, funding, and continuing operation with qualified personnel. Follow a disciplined roadmap.
- Baseline assessment. Gather historical data, do speciated arsenic testing, and characterize interferences. Map source chemistry and geology.
- Vertical profiling and pilot studies. Before constructing a production well or committing to treatment, pilot a representative treatment process at small scale. Validate preoxidation, contact times, media life, and backwash volumes under real water conditions and seasonal variability.
- Life cycle cost and funding. Calculate total cost of ownership — capital, chemical, labor, energy, residuals handling, and replacement media. Seek grants and loans with realistic operating cost projections.
- Construction and startup. Conduct construction with commissioning tests, perform startup training, and verify design assumptions with real-time performance data.
- Operational SOPs and training. Develop standard operating procedures, emergency procedures, monitoring schedules, and training programs. Operators should have hazardous material handling training and demonstrate competence with your specific treatment processes.
- Monitoring and continuous improvement. Track key performance indicators (KPIs): entry point arsenic concentration, media run length, chemical usage, backwash and residuals volumes, and cost-per-unit water treated. Create a dashboard with trend charts so operators and managers can spot changes early.

Pilot testing is vital — don’t skip it. Water chemistry that changes seasonally or that includes competing anions can drastically reduce media life or increase chemical consumption. Running a pilot for several months captures seasonality and provides realistic estimates for the full-scale plant.
Step 12: Final checklist and recommended priorities for small systems
When you are responsible for safe drinking water, prioritize practical, maintainable, and regulatory-compliant interventions. Here is a condensed checklist to carry forward with your project planning and operations.
- Baseline: obtain speciated arsenic and a full suite of chemistry tests including iron, manganese, silica, phosphate, alkalinity, pH, DO, and TOC.
- Source control first: perform vertical profiling and design wells to avoid high-arsenic horizons. Pilot test well design choices with a pilot or test well.
- Blending: evaluate blending before treatment if you have multiple sources. Do the math and confirm with seasonal data.
- Treatment selection: choose the simplest technology your operators can reliably run. For many small systems, coagulation/filtration or adsorptive media is appropriate. Preoxidation and pH control are commonly required.
- Residuals plan: nail down TCLP testing and disposal routes before equipment purchase. Include residuals transport and disposal in your O&M budget.
- Operator training: ensure staff are trained in chemical handling, media change-out, sampling, and hazardous waste procedures if applicable.
- Regulatory compliance: maintain sampling logs, consumer confidence report entries, and documented SOPs. If using blending or POU/POE, have documented agreements and monitoring plans.
- Funding and lifecycle: evaluate grants and loans but base decisions on life cycle costs rather than first cost alone.
- Monitoring and KPIs: build a dashboard for entry point arsenic, media runtime, chemical use, and residuals generation. Treat trends as early warning signals.

Simple maintainable solutions often outperform complex high-maintenance systems in small communities. Keep systems as straightforward as possible and make sure responsibilities and backups are clear. If full central treatment is required, build redundancy with lead/lag systems or parallel trains to prevent supply interruptions and avoid breakthrough surprises.
Closing notes: prioritizing prevention and planning for the long term
Arsenic is a naturally occurring challenge in groundwater but it is manageable with planning, data, and the right combination of source control and treatment. Start with the aquifer before you assume you will need a long-term treatment plant. Where source control is not enough, pick a technology that your operators can run reliably, and be explicit about the long-term costs associated with chemical use, media replacement, and residuals disposal.
When you design and operate an arsenic program, two themes should guide your decisions: prevention at the source and predictable, maintainable treatment. Do the geologic homework, profile vertically, test seasonally, pilot treatment, and manage residuals from day one. With that approach you will protect public health while keeping the project sustainable for your system.

If you need to prioritize immediate next steps for a community or well, do the following in order:
- Collect speciated arsenic and a full chemistry baseline.
- Conduct vertical profiling of the proposed or existing well field.
- Perform a pilot of the most likely treatment approach based on chemistry and operator capacity.
- Develop a residuals disposal plan and build lifecycle costs into funding requests.
- Train operators and set up KPI monitoring with trend dashboards.
Arsenic is manageable, but only if you treat it as a program rather than a single procurement task. Make decisions grounded in data and keep the system maintainable for the long term.


