Introduction to Manganese in Water Wells
Today we’re talking about manganese and water wells.
Welcome to this edition of Groundwater Talk Live, where we delve into the important topic of manganese in water wells. This discussion was prompted by a recent issue faced by a small system struggling with manganese contamination. Understanding manganese in groundwater is crucial, as it can pose significant challenges for water quality and management. In this session, we aim to explore the various aspects of manganese presence in water wells, providing insights and solutions to address these issues effectively.

In the following sections, we will cover the chemical properties of manganese, its health implications, and the role of bacteria in manganese accumulation. We will also discuss geological and environmental factors, the well health check process, and effective treatment and management strategies. Join us as we navigate through these critical topics to enhance your understanding and management of manganese in water wells.
Chemical Properties of Manganese
Manganese, a lesser-known but significant element, is element number 25 on the periodic table. It is positioned between chromium and iron, sharing the transitional metal category, which accounts for some of its chemical similarities with these elements.
“Manganese is element number twenty five on the periodic table.”
Manganese on the Periodic Table
Manganese is classified as a transitional metal, which means it shares common chemical characteristics with other elements in this category, particularly iron and chromium. This relationship is crucial as manganese and iron often occur together in natural settings, including water wells.
Chemical Similarities and Differences with Iron
While manganese and iron share many chemical properties due to their proximity on the periodic table, they also have distinct differences. These differences and similarities are important when considering their behavior in water wells and their accumulation processes.
Valence States and Their Significance
Manganese exhibits several valence states, notably +2, +3, +4, and +7, with +2 and +4 being the most common. The valence state of an element indicates its ability to accept electrons. For instance, in a +2 state, a manganese atom can accept two additional electrons, which is significant when it combines with other elements.
Oxidation Process in Water
The oxidation states of manganese play a critical role in its chemical reactions, especially in water. Understanding these states helps in predicting how manganese will interact with other substances in groundwater, which is essential for managing its presence in water wells.

Health Implications of Manganese in Groundwater
Manganese is considered an essential nutrient for humans, often found in dietary supplements. However, excessive levels, particularly in groundwater, can pose health risks. The Environmental Protection Agency (EPA) has established a secondary Maximum Contaminant Level (MCL) of 0.05 milligrams per liter for manganese. While this is not enforceable at the national level, California enforces this standard, making it a regulatory requirement within the state.
The EPA is considering promoting a primary MCL for manganese, which would be enforceable nationwide once approved. This process involves extensive toxicological evaluation and could take several years. In California, the distinction between secondary and primary MCLs is minimal since both are enforceable.
Manganese typically oxidizes in water, transitioning from a soluble form (Mn+2) to a stable compound (Mn+4), often seen as black manganese oxide deposits in wells. These accumulations can indicate manganese levels exceeding 0.1 milligrams per liter, leading to significant precipitation.

“Manganese is considered an essential nutrient for humans.”
Monitoring manganese levels in groundwater is crucial, especially as it tends to precipitate at lower concentrations compared to iron. The presence of manganese and iron often coincides, and their accumulation can be exacerbated by oxidizing bacteria, which will be discussed further in the section on Manganese Accumulation and Bacteria.
Manganese Accumulation and Bacteria
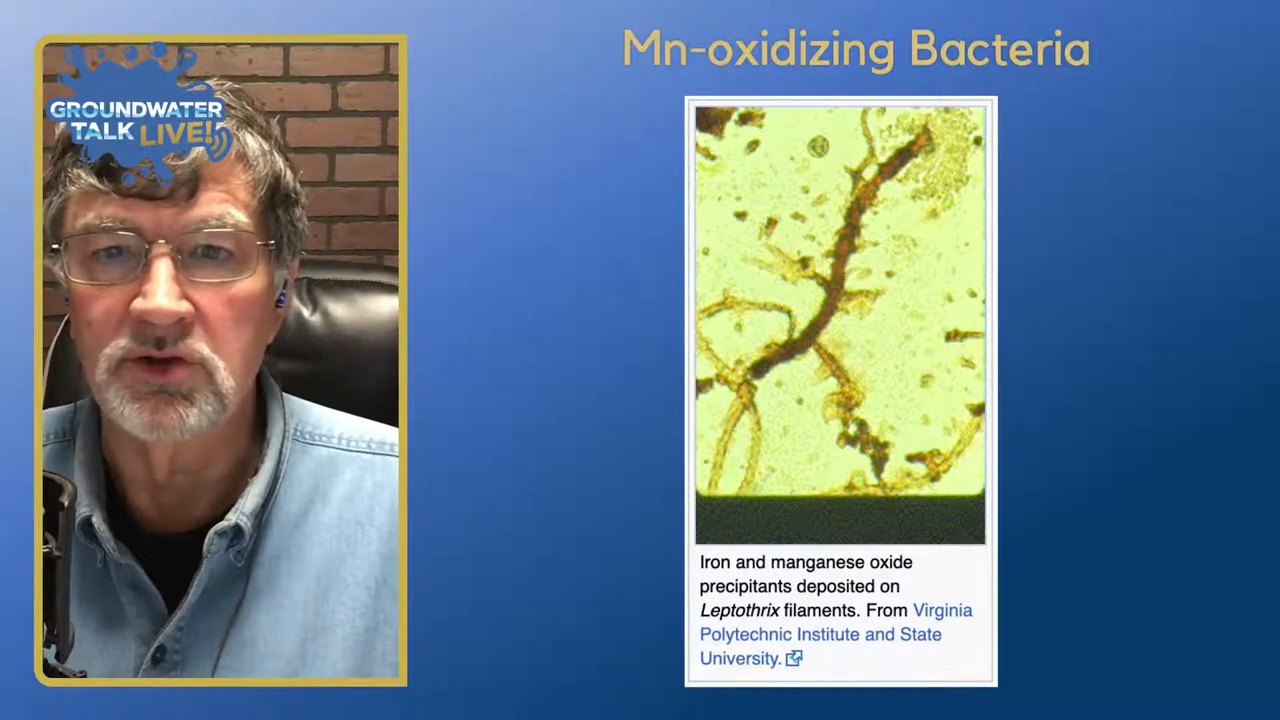
The role of bacteria in manganese accumulation is significant, particularly in the presence of iron and manganese oxidizing bacteria. These bacteria can enhance the potential for manganese to accumulate in water wells. The process involves biogenic components, where living organisms, such as bacteria, contribute to the formation and precipitation of manganese compounds.
“The presence of iron and manganese oxidizing bacteria can increase the potential for manganese accumulation.”
Biogenic Components and Their Impact
Biogenic action, primarily driven by bacteria, plays a crucial role in the oxidation and precipitation of manganese. Manganese in its soluble form, known as manganese plus two (Mn²⁺), is found in solution within wells. As it oxidizes, it transitions to manganese plus four (Mn⁴⁺), forming oxides, oxyhydroxides, and carbonates. This transformation is largely facilitated by bacteria, which can significantly influence the chemical state and accumulation of manganese in wells.
Interaction with Iron Oxidizing Bacteria
The interaction between manganese and iron oxidizing bacteria is a key factor in manganese accumulation. These bacteria thrive in specific environmental conditions, such as those with varying pH levels and oxidation-reduction potentials (ORP). In aerobic environments with high ORP, manganese tends to oxidize and precipitate, forming stable compounds like manganese dioxide. Conversely, in anaerobic environments with low ORP, manganese remains in its soluble form.
Understanding these interactions and the conditions that favor manganese accumulation is essential for managing and mitigating its presence in water wells. This knowledge is also vital for the well health check process, where the bacterial component is evaluated to assess the potential for manganese and other contaminants to accumulate.

Geological and Environmental Factors
The presence of manganese in water wells is influenced by various geological and environmental factors. Manganese naturally occurs in different geological settings, including dolomite, limestone, shales, igneous rocks, and ores like rhodochrosite. These rocks can leach manganese into groundwater, especially when manganese compounds oxidize in aqueous solutions.
“The USGS did a study a number of years ago and really this shows a map and the red obviously is population exposed to elevated manganese levels.”
Natural Occurrence and Distribution
Manganese is widely distributed across the United States, as shown in studies by the USGS and other regional research. For instance, a study in California from 2011 to 2019 found 435 sources with manganese levels above 0.5 mg/L, affecting 322 water systems across 47 of the 54 counties. This indicates a widespread distribution influenced by natural geological formations.
In Georgia, manganese levels exceeding the EPA’s secondary MCL were observed, particularly in regions with specific geological characteristics like the Blue Ridge and limestone valleys. These areas have higher manganese concentrations due to the natural substitution of calcium and manganese in rocks.
Influence of Soil and Organic Matter
Soil composition and organic matter also play a significant role in manganese levels in groundwater. Organic compounds, such as humic and fulvic acids, can mobilize manganese, increasing its concentration in water wells. This is particularly relevant in areas with high organic content in the soil.
Regional Studies and Findings
Regional studies highlight the impact of geology on manganese distribution. In North Carolina, the USGS found high manganese levels in the Carolina slate belt and Triassic basin, where geological formations contribute to elevated concentrations in residential wells. These findings underscore the importance of geological factors in determining manganese levels in groundwater.
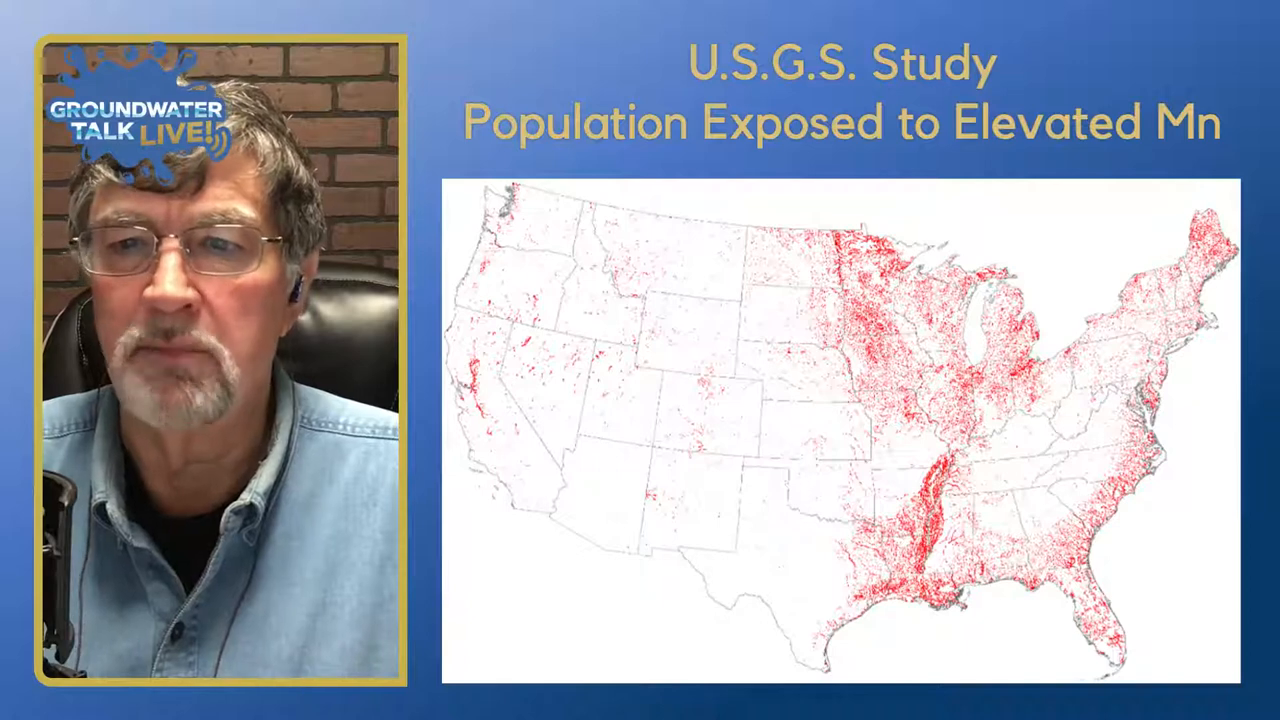
These studies illustrate that while manganese is naturally occurring and widely distributed, specific geological and environmental conditions can lead to higher concentrations in certain areas. Understanding these factors is crucial for managing manganese levels in water wells.
Well Health Check Process
The well health check process is a crucial step in maintaining the integrity and functionality of your water well. It involves a comprehensive examination of the well’s chemistry and physical condition to ensure its optimal performance and longevity.
“The well health check process is looking at the chemistry in your well.”
Importance of Well Health Checks
Regular well health checks are essential for identifying potential issues that could lead to corrosion, metal accumulation, or elevated concentrations of harmful compounds. By understanding the specific conditions within your well, you can develop a tailored rehabilitation program to address any problems effectively.
Components of the Well Health Check Process
- Chemical Analysis: This involves breaking down elements such as iron and manganese into their various states (e.g., iron two, iron three, manganese two, manganese four) to understand their impact on the well.
- Bacteria Load Assessment: Identifying the types and loads of bacteria present in the well can help pinpoint causes of corrosion and accumulation issues.
- Well Video Inspection: Using video technology to visually inspect the well can reveal the extent and type of accumulations, such as manganese (black to dark brown), iron (orange to lighter brown), and calcium carbonate (white to tan).
- Incrustation Sampling: Although often unnecessary, sampling encrustations from the well’s sidewalls can provide additional confirmation of the well’s condition. This is typically done when the pump is removed for maintenance.
How to Assess Manganese Levels in Wells
Assessing manganese levels involves both chemical analysis and visual inspection. The presence of manganese-related bacteria can be indicated through tests like the IRB bark test, which is cost-effective and provides valuable insights.
Conclusion
By conducting a thorough well health check, you can gain a comprehensive understanding of your well’s condition, allowing for informed decisions on maintenance and rehabilitation. This proactive approach helps prevent long-term issues and ensures the well’s continued efficiency and safety.
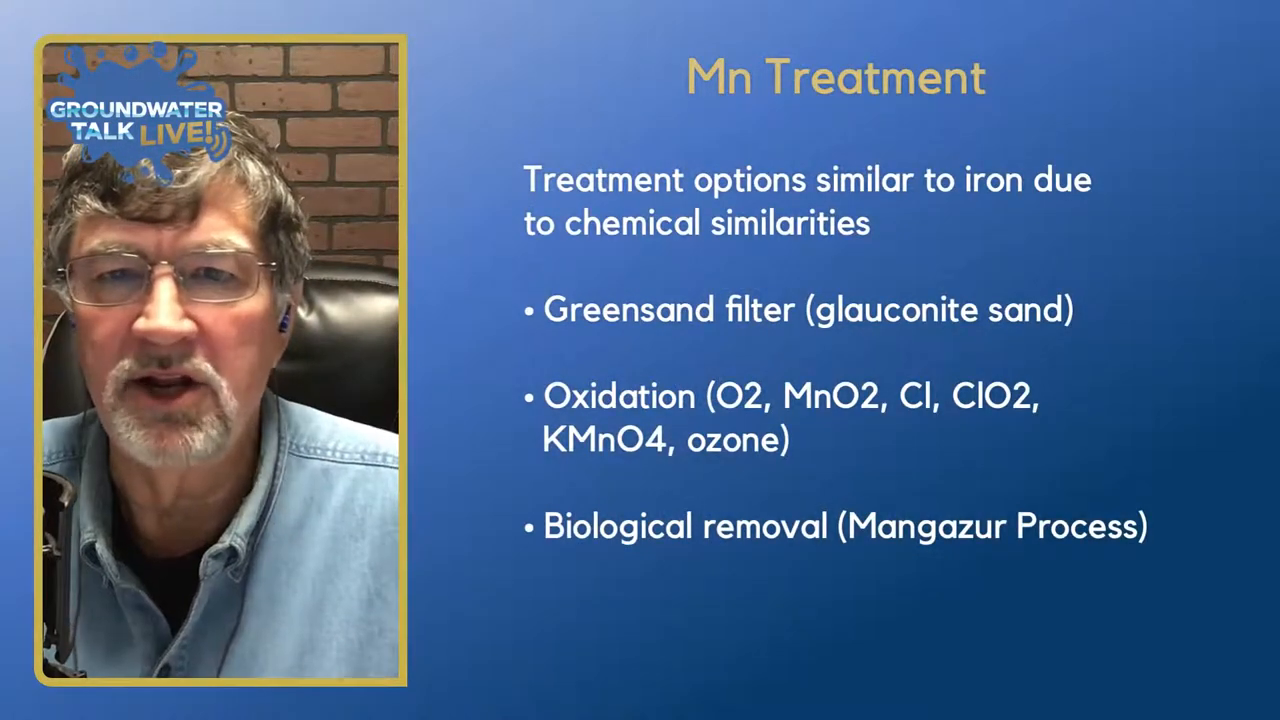
Treatment and Management of Manganese in Water Wells
Treatment options for manganese in water wells are quite similar to those for iron, due to their chemical similarities. The primary method employed is the use of a greensand filter, which utilizes glauconite sand to effectively filter out both iron and manganese. This method is particularly effective when the levels of these metals are not excessively high.
“Treatment options very similar to iron due to the chemical similarities.”
In addition to greensand filters, active oxidation treatments can be used. The goal of oxidation is to convert manganese from its soluble form (manganese two) to a stable compound (manganese four) that can be filtered out as it precipitates. Various oxidation techniques include the use of oxygen, manganese dioxide, chlorine, chlorine dioxide, potassium permanganate, and ozone. These methods are applied as part of the water treatment process, rather than directly in the well.
Another approach is the biological removal process known as the Mangazur process. This method employs specific bacteria to oxidize manganese, offering a biological alternative to chemical oxidation. The Mangazur process is patented and available through certain vendors, showing promising results in some cases.
Recent research from 2009-2010 has highlighted the potential for microbial-catalyzed manganese oxidation and reduction to occur simultaneously in environments with significant oxygen and chlorine levels. This finding, primarily observed in water treatment plants, has implications for wells and drinking water systems, suggesting that traditional strategies using chlorine and oxidizing conditions may need reevaluation. The study indicates that soluble manganese can still be present even in the presence of chlorine and oxygen, which is a concern for water treatment strategies.
Conclusion and Future Considerations
In conclusion, the discussion on manganese in water wells highlights the critical role of understanding both the chemical and microbiological components involved. As emphasized, “the chemistry in your well really makes a difference in a lot of these things,” indicating that a tailored approach is essential for effective management. There is no universal solution, and recognizing the unique conditions of each well is crucial.
Future considerations should focus on further exploring the microbiological aspects, as they significantly impact well health. The well health check process is a valuable tool in this regard, offering insights into the specific needs of each well.
Looking ahead, continued research and dialogue within the water community are vital. Engaging with platforms like Groundwater Talk Live can facilitate the exchange of knowledge and experiences, benefiting all stakeholders involved in groundwater management.


